当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NLRP3 inflammasome mediates pyroptosis of alveolar macrophages to induce radiation lung injury
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-01 , DOI: 10.1016/j.jhazmat.2024.136740 Mingwei Zhang, Hailin Lan, Meina Jiang, Minghuan Yang, Hongquan Chen, Shaoli Peng, Xuezhen Wang, Yarui Zhang, Xingxin Huang, Lianhuang Li, Chun Chen, Jinsheng Hong
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-12-01 , DOI: 10.1016/j.jhazmat.2024.136740 Mingwei Zhang, Hailin Lan, Meina Jiang, Minghuan Yang, Hongquan Chen, Shaoli Peng, Xuezhen Wang, Yarui Zhang, Xingxin Huang, Lianhuang Li, Chun Chen, Jinsheng Hong
|
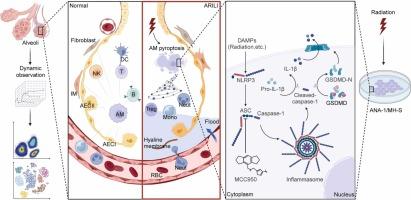
Alveolar macrophages play a crucial role in maintaining lung homeostasis. However, the mechanisms underlying alveolar macrophage pyroptosis and inflammasome activation in radiation-induced lung injury remain unclear. In this study, we employed multicolor flow cytometry and single-cell RNA sequencing to reveal the immune cell and cell death landscape in the tissue microenvironment of radiation-induced lung injury. Additionally, we utilized mass spectrometry, co-immunoprecipitation and Duolink techniques to investigate the core inflammasome responsible for mediating alveolar macrophage pyroptosis. We noticed that the percentage of alveolar macrophages, T, B and epithelial cells decreased significantly post-irradiation. Notably, the proportional changes in alveolar macrophages closely correlated with Szapiels' pneumonia score. Furthermore, alveolar macrophages emerged as the earliest cell type to initiate pyroptosis and act as pivotal regulators of cell communication. In vitro and in vivo experiments, we observed a significant increase in NLRP3 binding to the apoptosis-associated speck-like protein in irradiated alveolar macrophages. In vivo , MCC950 effectively inhibited alveolar macrophage pyroptosis and significantly reducing inflammatory cells recruitment. Subsequently, targeting AM pyroptosis ultimately inhibit the infiltration of interstitial macrophages and the activation of fibroblasts, decrease collagen deposition and alleviate the severity of radiation-induced lung fibrosis. Targeting alveolar macrophage pyroptosis and NLRP3 inflammasome activation hold substantial therapeutic potential for mitigating radiation-induced lung injury.
中文翻译:

NLRP3 炎性小体介导肺泡巨噬细胞焦亡以诱导放射性肺损伤
肺泡巨噬细胞在维持肺稳态中起着至关重要的作用。然而,辐射诱导的肺损伤中肺泡巨噬细胞焦亡和炎性小体激活的机制仍不清楚。在这项研究中,我们采用多色流式细胞术和单细胞 RNA 测序来揭示辐射诱导肺损伤的组织微环境中的免疫细胞和细胞死亡景观。此外,我们利用质谱、免疫共沉淀和 Duolink 技术来研究负责介导肺泡巨噬细胞焦亡的核心炎性小体。我们注意到照射后肺泡巨噬细胞、 T 、 B 和上皮细胞的百分比显着降低。值得注意的是,肺泡巨噬细胞的比例变化与 Szapiels 的肺炎评分密切相关。此外,肺泡巨噬细胞成为最早启动细胞焦亡的细胞类型,并作为细胞通讯的关键调节因子。在体外和体内实验中,我们观察到 NLRP3 与照射肺泡巨噬细胞中细胞凋亡相关斑点样蛋白的结合显着增加。在体内,MCC950 有效抑制肺泡巨噬细胞焦亡并显着减少炎症细胞募集。随后,靶向 AM 焦亡最终抑制间质巨噬细胞的浸润和成纤维细胞的激活,减少胶原蛋白沉积并减轻辐射诱导的肺纤维化的严重程度。靶向肺泡巨噬细胞焦亡和 NLRP3 炎性小体激活在减轻辐射诱导的肺损伤方面具有巨大的治疗潜力。
更新日期:2024-12-01
中文翻译:

NLRP3 炎性小体介导肺泡巨噬细胞焦亡以诱导放射性肺损伤
肺泡巨噬细胞在维持肺稳态中起着至关重要的作用。然而,辐射诱导的肺损伤中肺泡巨噬细胞焦亡和炎性小体激活的机制仍不清楚。在这项研究中,我们采用多色流式细胞术和单细胞 RNA 测序来揭示辐射诱导肺损伤的组织微环境中的免疫细胞和细胞死亡景观。此外,我们利用质谱、免疫共沉淀和 Duolink 技术来研究负责介导肺泡巨噬细胞焦亡的核心炎性小体。我们注意到照射后肺泡巨噬细胞、 T 、 B 和上皮细胞的百分比显着降低。值得注意的是,肺泡巨噬细胞的比例变化与 Szapiels 的肺炎评分密切相关。此外,肺泡巨噬细胞成为最早启动细胞焦亡的细胞类型,并作为细胞通讯的关键调节因子。在体外和体内实验中,我们观察到 NLRP3 与照射肺泡巨噬细胞中细胞凋亡相关斑点样蛋白的结合显着增加。在体内,MCC950 有效抑制肺泡巨噬细胞焦亡并显着减少炎症细胞募集。随后,靶向 AM 焦亡最终抑制间质巨噬细胞的浸润和成纤维细胞的激活,减少胶原蛋白沉积并减轻辐射诱导的肺纤维化的严重程度。靶向肺泡巨噬细胞焦亡和 NLRP3 炎性小体激活在减轻辐射诱导的肺损伤方面具有巨大的治疗潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号