当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1-Hydroxy(and 1-Alkoxy, 1-Acyloxy)-1H-indoles and evaluations of their suppressive activities against tumor growth through inhibiting lactate dehydrogenase A
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-30 , DOI: 10.1016/j.ejmech.2024.117104
Se-Yun Cheon 1 , Ye Eun Kim 2 , Eun-Sun Yang 1 , Yoo Jin Lim 2 , Chang-Hwan Bae 3 , Jung-Sook Jin 1 , Wonyoung Park 4 , Bo-Sung Kim 4 , Chorong Kim 2 , Hyunsung Cho 2 , Seungtae Kim 4 , Sang Hyup Lee 2 , Ki-Tae Ha 4
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-30 , DOI: 10.1016/j.ejmech.2024.117104
Se-Yun Cheon 1 , Ye Eun Kim 2 , Eun-Sun Yang 1 , Yoo Jin Lim 2 , Chang-Hwan Bae 3 , Jung-Sook Jin 1 , Wonyoung Park 4 , Bo-Sung Kim 4 , Chorong Kim 2 , Hyunsung Cho 2 , Seungtae Kim 4 , Sang Hyup Lee 2 , Ki-Tae Ha 4
Affiliation
|
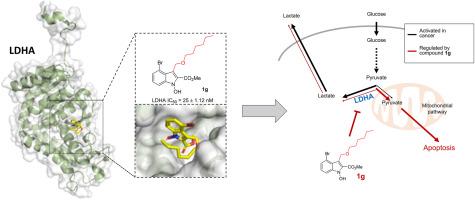
Inhibition of lactate dehydrogenase (LDH) has emerged as a promising cancer therapy strategy due to its essential role in the metabolic transformation of cancer cells. In this study, 53 derivatives of 1-hydroxy(and 1-alkoxy, 1-acyloxy)indoles were designed, synthesized, and biologically evaluated. Several multi-substituted 1-hydroxy(and 1-alkoxy, 1-acyloxy)indole compounds exhibited inhibitory activity against the LDH-A isoform (LDHA). We confirmed that the C(3)-substituent provided additional significant hydrogen bonding and hydrophobic interactions, which enhanced the LDHA inhibitory activity with high selectivity. Our results revealed that methyl 4-bromo-3-[(n -hexyloxy)methyl]-1-hydroxy-1H -indole-2-carboxylate (1g ), selectively inhibited LDHA (IC50 = 25 ± 1.12 nM) without affecting the LDH-B isoform (LDHB). The compound exhibited potent cytotoxic activity in several cancer cell lines, including DLD-1 colorectal cancer cells (GI50 = 27 ± 1.2 μM). Compound 1g significantly inhibited cancer cell growth by activating apoptotic pathways in a xenograft cancer model, without causing weight loss or liver and kidney damage. Therefore, compound 1g may serve as a highly specific and promising candidate for the development of LDHA inhibitors for cancer therapy.
中文翻译:

1-羟基(和 1-烷氧基,1-酰氧基)-1H-吲哚的合成及其通过抑制乳酸脱氢酶 A 对肿瘤生长的抑制活性的评价
抑制乳酸脱氢酶 (LDH) 已成为一种很有前途的癌症治疗策略,因为它在癌细胞的代谢转化中起着重要作用。在这项研究中,设计、合成了 1-羟基(和 1-烷氧基、1-酰氧基)吲哚的 53 种衍生物,并进行了生物学评价。几种多取代的 1-羟基(和 1-烷氧基,1-酰氧基)吲哚化合物对 LDH-A 亚型 (LDHA) 表现出抑制活性。我们证实 C(3) -取代基提供了额外的显着氢键和疏水相互作用,从而以高选择性增强了 LDHA 抑制活性。我们的结果显示,4-溴-3-[(正己氧基)甲基]-1-羟基-1H-吲哚-2-羧酸甲酯 (1g) 选择性抑制 LDHA (IC50 = 25 ± 1.12 nM),而不影响 LDH-B 亚型 (LDHB)。该化合物在多种癌细胞系中表现出有效的细胞毒活性,包括 DLD-1 结直肠癌细胞 (GI50 = 27 ± 1.2 μM)。化合物 1g 通过激活异种移植癌症模型中的凋亡途径显着抑制癌细胞生长,而不会导致体重减轻或肝肾损伤。因此,化合物 1g 可作为开发用于癌症治疗的 LDHA 抑制剂的高度特异性和有前途的候选药物。
更新日期:2024-11-30
中文翻译:

1-羟基(和 1-烷氧基,1-酰氧基)-1H-吲哚的合成及其通过抑制乳酸脱氢酶 A 对肿瘤生长的抑制活性的评价
抑制乳酸脱氢酶 (LDH) 已成为一种很有前途的癌症治疗策略,因为它在癌细胞的代谢转化中起着重要作用。在这项研究中,设计、合成了 1-羟基(和 1-烷氧基、1-酰氧基)吲哚的 53 种衍生物,并进行了生物学评价。几种多取代的 1-羟基(和 1-烷氧基,1-酰氧基)吲哚化合物对 LDH-A 亚型 (LDHA) 表现出抑制活性。我们证实 C(3) -取代基提供了额外的显着氢键和疏水相互作用,从而以高选择性增强了 LDHA 抑制活性。我们的结果显示,4-溴-3-[(正己氧基)甲基]-1-羟基-1H-吲哚-2-羧酸甲酯 (1g) 选择性抑制 LDHA (IC50 = 25 ± 1.12 nM),而不影响 LDH-B 亚型 (LDHB)。该化合物在多种癌细胞系中表现出有效的细胞毒活性,包括 DLD-1 结直肠癌细胞 (GI50 = 27 ± 1.2 μM)。化合物 1g 通过激活异种移植癌症模型中的凋亡途径显着抑制癌细胞生长,而不会导致体重减轻或肝肾损伤。因此,化合物 1g 可作为开发用于癌症治疗的 LDHA 抑制剂的高度特异性和有前途的候选药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号