当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Parasitic Products Formed during Discharge Limit Capacity and Rechargeability in Li–O2 Cells
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-11-30 , DOI: 10.1021/acsenergylett.4c03142 Akhila Subhakumari, Telna Thomas, Naga Phani B Aetukuri
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-11-30 , DOI: 10.1021/acsenergylett.4c03142 Akhila Subhakumari, Telna Thomas, Naga Phani B Aetukuri
|
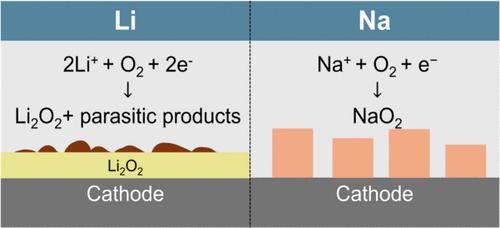
Aprotic metal–oxygen batteries, especially Li–O2 and Na–O2 batteries, are considered high energy density alternatives to conventional Li-ion batteries. However, the rechargeability and, consequently, the cycle life of the metal–oxygen batteries are poor. In general, the poor rechargeability of these batteries is attributed to the oxidative instabilities of the carbon cathode and aprotic electrolytes at high oxidative potentials during charge. In this work, we employ complementary measurements, including electrochemical impedance spectroscopy, distribution of relaxation times analysis, chemical titrations of discharge products, and differential electrochemical mass spectrometry measurements to investigate electrochemical processes that limit rechargeability in these chemistries. Contrary to the extant understanding, our analysis shows that the origin of recharge inefficiencies in Li–O2 cells is the formation of parasitic side products during discharge. Significantly, our results suggest that cathode passivation by Li2O2 is not capacity-limiting during discharge, suggesting that increased capacities and rechargeability should be simultaneously possible in Li–O2 batteries.
中文翻译:

放电过程中形成的寄生产物限制了 Li-O2 电池的容量和可充电性
非质子金属-氧电池,尤其是 Li-O2 和 Na-O2 电池,被认为是传统锂离子电池的高能量密度替代品。然而,金属氧电池的可充电性以及因此的循环寿命很差。一般来说,这些电池的可充电性差是由于充电过程中碳阴极和非质子电解质在高氧化电位下的氧化不稳定性。在这项工作中,我们采用互补测量,包括电化学阻抗谱、弛豫时间分布分析、放电产物的化学滴定和差分电化学质谱测量,以研究限制这些化学物质可充电性的电化学过程。与现有的理解相反,我们的分析表明,Li-O2 电池充电效率低下的根源是在放电过程中形成寄生副产物。值得注意的是,我们的结果表明,Li2O2 的阴极钝化在放电过程中没有容量限制,这表明在 Li-O2 电池中应该可以同时增加容量和可充电性。
更新日期:2024-11-30
中文翻译:

放电过程中形成的寄生产物限制了 Li-O2 电池的容量和可充电性
非质子金属-氧电池,尤其是 Li-O2 和 Na-O2 电池,被认为是传统锂离子电池的高能量密度替代品。然而,金属氧电池的可充电性以及因此的循环寿命很差。一般来说,这些电池的可充电性差是由于充电过程中碳阴极和非质子电解质在高氧化电位下的氧化不稳定性。在这项工作中,我们采用互补测量,包括电化学阻抗谱、弛豫时间分布分析、放电产物的化学滴定和差分电化学质谱测量,以研究限制这些化学物质可充电性的电化学过程。与现有的理解相反,我们的分析表明,Li-O2 电池充电效率低下的根源是在放电过程中形成寄生副产物。值得注意的是,我们的结果表明,Li2O2 的阴极钝化在放电过程中没有容量限制,这表明在 Li-O2 电池中应该可以同时增加容量和可充电性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号