当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical and Experimental Analysis of Pb (II) Ion Adsorption Using Surface Modified Macroalgal Biosorbents: Modelling and Desorption Study
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-30 , DOI: 10.1021/acs.iecr.4c02591 P Thamarai, S. Karishma, V.C. Deivayanai, A. Saravanan, P R Yaashikaa
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-30 , DOI: 10.1021/acs.iecr.4c02591 P Thamarai, S. Karishma, V.C. Deivayanai, A. Saravanan, P R Yaashikaa
|
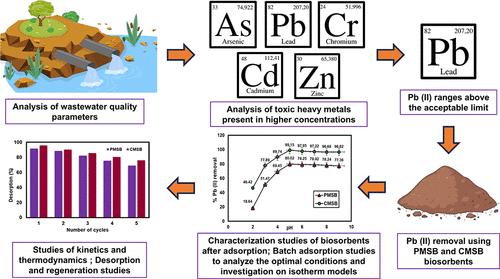
The critical issue of lead pollution in wastewater, which, even in low quantities, presents serious health risks, is the focus of this investigation. The study investigates the adsorption capacities of Physically Modified Seaweed Biosorbent (PMSB) and Chemically Modified Seaweed Biosorbent (CMSB) for Pb (II) ion removal. SEM, EDX, FTIR, and XRD techniques were used to analyze surface morphology, elemental composition, functional groups, and crystallographic structure. Furthermore, it assesses the effect of pH, biosorbent dosage, temperature, initial Pb (II) ion concentration, and contact time on adsorption efficiency. The results indicated that the optimal parameters were 303 K in temperature, 5.0 in pH, and 1 g/L and 2.5 g/L of biosorbent for CMSB and PMSB, respectively, with contact durations of 40 and 80 min. The Freundlich isotherm model indicated adsorption on heterogeneous surfaces, with maximum adsorption capacities of 149.8 mg/g for PMSB and 175.5 mg/g for CMSB, demonstrating efficient Pb (II) ion removal. Higher R2 values from kinetic investigations indicate that the pseudo-first-order model fits PMSB and CMSB better for the adsorption of Pb (II) ions. The thermodynamic analysis found negative ΔH° and ΔG° values, indicating an exothermic and spontaneous adsorption mechanism, respectively. Desorption tests showed that CMSB retains greater efficiency across several cycles, demonstrating its durability and adaptability for long-term use. According to the studies, chemical modifications significantly enhance CMSB’s adsorption stability and effectiveness, which makes it a viable option for eliminating Pb (II) ions from wastewater and improving water quality.
中文翻译:

使用表面改性大型藻类生物吸附剂对 Pb (II) 离子吸附的理论和实验分析:建模和解吸研究
废水中的铅污染这一关键问题是本次调查的重点,即使铅污染量少,也会带来严重的健康风险。该研究调查了物理改性海藻生物吸附剂 (PMSB) 和化学改性海藻生物吸附剂 (CMSB) 去除 Pb (II) 离子的吸附能力。SEM、EDX、FTIR 和 XRD 技术用于分析表面形貌、元素组成、官能团和晶体结构。此外,它还评估了 pH 值、生物吸附剂剂量、温度、初始 Pb (II) 离子浓度和接触时间对吸附效率的影响。结果表明,CMSB 和 PMSB 的最佳参数分别为温度 303 K、pH 值 5.0、生物吸附剂 1 g/L 和 2.5 g/L,接触持续时间分别为 40 和 80 min。Freundlich 等温线模型表明在非均相表面上的吸附,PMSB 的最大吸附容量为 149.8 mg/g,CMSB 的最大吸附容量为 175.5 mg/g,表明 Pb (II) 离子有效去除。动力学研究中较高的 R2 值表明,伪一级模型更适合 PMSB 和 CMSB 对 Pb (II) 离子的吸附。热力学分析发现 ΔH° 和 ΔG° 值为负,分别表明放热和自发吸附机制。脱附测试表明,CMSB 在多个循环中保持更高的效率,证明了其耐用性和长期使用的适应性。根据研究,化学改性显著提高了 CMSB 的吸附稳定性和有效性,这使其成为从废水中去除 Pb (II) 离子和改善水质的可行选择。
更新日期:2024-11-30
中文翻译:

使用表面改性大型藻类生物吸附剂对 Pb (II) 离子吸附的理论和实验分析:建模和解吸研究
废水中的铅污染这一关键问题是本次调查的重点,即使铅污染量少,也会带来严重的健康风险。该研究调查了物理改性海藻生物吸附剂 (PMSB) 和化学改性海藻生物吸附剂 (CMSB) 去除 Pb (II) 离子的吸附能力。SEM、EDX、FTIR 和 XRD 技术用于分析表面形貌、元素组成、官能团和晶体结构。此外,它还评估了 pH 值、生物吸附剂剂量、温度、初始 Pb (II) 离子浓度和接触时间对吸附效率的影响。结果表明,CMSB 和 PMSB 的最佳参数分别为温度 303 K、pH 值 5.0、生物吸附剂 1 g/L 和 2.5 g/L,接触持续时间分别为 40 和 80 min。Freundlich 等温线模型表明在非均相表面上的吸附,PMSB 的最大吸附容量为 149.8 mg/g,CMSB 的最大吸附容量为 175.5 mg/g,表明 Pb (II) 离子有效去除。动力学研究中较高的 R2 值表明,伪一级模型更适合 PMSB 和 CMSB 对 Pb (II) 离子的吸附。热力学分析发现 ΔH° 和 ΔG° 值为负,分别表明放热和自发吸附机制。脱附测试表明,CMSB 在多个循环中保持更高的效率,证明了其耐用性和长期使用的适应性。根据研究,化学改性显著提高了 CMSB 的吸附稳定性和有效性,这使其成为从废水中去除 Pb (II) 离子和改善水质的可行选择。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号