Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removal of basic yellow dye molecules with chitosan-based magnetic field-sensitive particles from the aqueous solution
Polymer ( IF 4.1 ) Pub Date : 2024-11-30 , DOI: 10.1016/j.polymer.2024.127895 Ömer İpek, Şeyda Taşar, Neslihan Duranay
Polymer ( IF 4.1 ) Pub Date : 2024-11-30 , DOI: 10.1016/j.polymer.2024.127895 Ömer İpek, Şeyda Taşar, Neslihan Duranay
|
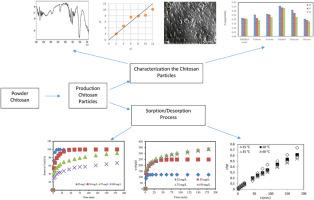
The study aimed to develop chitosan-based particle sorbents and evaluate their efficiency in removing reactive dyes from wastewater, leveraging chitosan's hydrophilic and positively charged properties. Polymeric particles were synthesized using a precipitation-aggregation method and characterized via analytical techniques. Sorption performance was tested using Basic Yellow 28 (BY28) dye under varying conditions of temperature, pH, sorbent dosage, dye concentration, and contact time. Optimal conditions were identified as 40 °C, pH 7, 0.2 g/L sorbent dosage, 75 mg/L dye concentration, and 180 min contact time, achieving a maximum sorption capacity of 330.96 mg/g. Experimental data were evaluated using isotherm models (Langmuir, Freundlich, Temkin, Dubinin-Radushkevich), with the Langmuir isotherm showing an R2 value close to one (0.998), indicating a strong fit. The theoretical maximum sorption capacity (qmax ) ranging from 196.08 to 322.58 mg/g across different conditions. Kinetic studies revealed the pseudo-second-order model best described the sorption process, with maximum sorption capacities (qe,c ) at different temperatures calculated at 243.90, 294.12, 312.50, and 333.33 mg/g at 25, 30, 35, and 40 °C, respectively. The endothermic nature of adsorption indicates improved efficiency at higher temperatures, aligning with industrial requirements. The study highlights the versatility of chitosan particles across a wide range of pH and temperatures, combining chemical and physical adsorption mechanisms. These particles demonstrate stability, efficiency, and eco-friendliness, making them suitable for sustainable water treatment. The findings reinforce the applicability of chitosan in addressing textile wastewater challenges, offering insights into optimization and scalability for industrial effluent management. The robustness of chitosan particles further underscores their potential for broader applications in wastewater treatment.
中文翻译:

用壳聚糖基磁场敏感颗粒从水溶液中去除碱性黄色染料分子
该研究旨在开发壳聚糖基颗粒吸附剂,并评估其利用壳聚糖的亲水性和带正电荷的特性从废水中去除活性染料的效率。使用沉淀聚集法合成聚合物颗粒,并通过分析技术进行表征。使用碱性黄 28 (BY28) 染料在不同温度、pH 值、吸附剂剂量、染料浓度和接触时间条件下测试吸附性能。确定最佳条件为 40 °C、pH 7、0.2 g/L 吸附剂用量、染料浓度 75 mg/L 和接触时间 180 min,最大吸附容量为 330.96 mg/g。使用等温线模型 (Langmuir, Freundlich, Temkin, Dubinin-Radushkevich) 评估实验数据,其中 Langmuir 等温线显示 R2 值接近 1 (0.998),表明拟合性强。在不同条件下,理论最大吸附量 (qmax) 范围为 196.08 至 322.58 mg/g。动力学研究表明,伪二级模型最能描述吸附过程,在 25、30、35 和 40 °C 下,不同温度下的最大吸附容量 (qe,c) 分别为 243.90、294.12、312.50 和 333.33 mg/g。吸附的吸热性质表明在较高温度下效率更高,符合工业要求。该研究强调了壳聚糖颗粒在较宽的 pH 值和温度范围内的多功能性,结合了化学和物理吸附机制。这些颗粒表现出稳定性、效率和环保性,使其适用于可持续的水处理。 这些发现加强了壳聚糖在应对纺织废水挑战方面的适用性,为工业废水管理的优化和可扩展性提供了见解。壳聚糖颗粒的稳健性进一步强调了它们在废水处理中更广泛应用的潜力。
更新日期:2024-11-30
中文翻译:

用壳聚糖基磁场敏感颗粒从水溶液中去除碱性黄色染料分子
该研究旨在开发壳聚糖基颗粒吸附剂,并评估其利用壳聚糖的亲水性和带正电荷的特性从废水中去除活性染料的效率。使用沉淀聚集法合成聚合物颗粒,并通过分析技术进行表征。使用碱性黄 28 (BY28) 染料在不同温度、pH 值、吸附剂剂量、染料浓度和接触时间条件下测试吸附性能。确定最佳条件为 40 °C、pH 7、0.2 g/L 吸附剂用量、染料浓度 75 mg/L 和接触时间 180 min,最大吸附容量为 330.96 mg/g。使用等温线模型 (Langmuir, Freundlich, Temkin, Dubinin-Radushkevich) 评估实验数据,其中 Langmuir 等温线显示 R2 值接近 1 (0.998),表明拟合性强。在不同条件下,理论最大吸附量 (qmax) 范围为 196.08 至 322.58 mg/g。动力学研究表明,伪二级模型最能描述吸附过程,在 25、30、35 和 40 °C 下,不同温度下的最大吸附容量 (qe,c) 分别为 243.90、294.12、312.50 和 333.33 mg/g。吸附的吸热性质表明在较高温度下效率更高,符合工业要求。该研究强调了壳聚糖颗粒在较宽的 pH 值和温度范围内的多功能性,结合了化学和物理吸附机制。这些颗粒表现出稳定性、效率和环保性,使其适用于可持续的水处理。 这些发现加强了壳聚糖在应对纺织废水挑战方面的适用性,为工业废水管理的优化和可扩展性提供了见解。壳聚糖颗粒的稳健性进一步强调了它们在废水处理中更广泛应用的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号