当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing Lithium Ion Conduction in LLZO-Based Solid Electrolytes through Anion Doping for Advanced Energy Storage: Insights from Molecular Dynamics Simulations
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-11-29 , DOI: 10.1021/acs.chemmater.4c02506 Cristina Lopez-Puga, Jincheng Du
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-11-29 , DOI: 10.1021/acs.chemmater.4c02506 Cristina Lopez-Puga, Jincheng Du
|
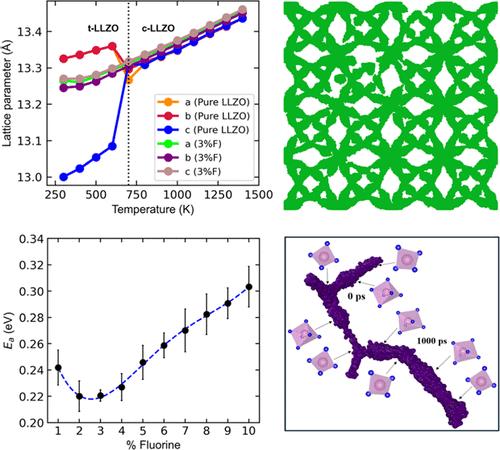
Solid-state electrolytes (SSEs) have emerged as promising alternatives to traditional liquid electrolytes due to their enhanced safety, higher stability and energy density in energy storage applications. Among SSEs, cubic Li7La3Zr2O12 (LLZO) is considered particularly promising, offering high lithium ion conductivity, high chemical stability to metal anode and a wide electrochemical stability window. Nevertheless, the cubic phase converts to a less conductive tetragonal phase during cooling in pure LLZO. Doping is one of most effective methods to stabilize the cubic LLZO at lower temperatures and improve the ion conductivity. While there is extensive research on cation site substitutions, studies on anion doping are very limited. We have investigated the effects of fluorine doping on the phase stability and ion conductivity of LLZO, exploring fluorine concentrations ranging from 1 to 10% across a wide temperature range of 300–1400 K using molecular dynamics (MD) simulations based on polarizable shell model potentials. Our results indicate that 3% fluorine doping achieves the highest diffusion coefficient (3.69 × 10–7 cm2 s–1) at room temperature, while the lowest activation energy (∼0.22 eV) also occurs at around 3% doping, which is in good agreement with experimental observations. Doping at 1% was found to be insufficient to stabilize the cubic phase, while high fluorine concentrations (>4%) inhibited ion migration pathways due to stronger electrostatic interactions between point defects VLi′ and FO•. Defect formation energies were also calculated to study defect formation and interactions and their effect on lithium ion conduction. Lithium ion diffusion pathways and mechanisms are also explored by using trajectories from MD simulations. This study provides insights into the optimization of fluorine-doped LLZO, suggesting that moderate doping levels (around 3%) offer a balance between phase stability and ionic conductivity.
中文翻译:

通过阴离子掺杂增强基于 LLZO 的固体电解质中的锂离子传导以实现高级储能:来自分子动力学模拟的见解
固态电解质 (SSE) 因其在储能应用中具有增强的安全性、更高的稳定性和能量密度,已成为传统液体电解质的有前途的替代品。在 SSE 中,立方锂7La3Zr2O12 (LLZO) 被认为特别有前途,具有高锂离子电导率、对金属阳极的高化学稳定性和较宽的电化学稳定性窗口。然而,在纯 LLZO 中冷却过程中,立方相会转化为导电性较低的四方相。掺杂是在较低温度下稳定立方 LLZO 并提高离子电导率的最有效方法之一。虽然对阳离子位点取代进行了广泛的研究,但对阴离子掺杂的研究非常有限。我们研究了氟掺杂对 LLZO 相稳定性和离子电导率的影响,使用基于可极化壳模型电位的分子动力学 (MD) 模拟,在 300-1400 K 的宽温度范围内探索了 1% 至 10% 的氟浓度。我们的结果表明,3% 氟掺杂在室温下达到最高的扩散系数 (3.69 × 10-7 cm2 s–1),而最低的活化能 (∼0.22 eV) 也出现在 3% 左右的掺杂下,这与实验观察结果非常吻合。发现 1% 的掺杂不足以稳定立方相,而高氟浓度 (>4%) 由于点缺陷 VLi 和 FO• 之间更强的静电相互作用而抑制了离子迁移途径。还计算了缺陷形成能量以研究缺陷形成和相互作用及其对锂离子传导的影响。 还通过使用 MD 模拟的轨迹来探索锂离子扩散途径和机制。这项研究为氟掺杂 LLZO 的优化提供了见解,表明适度的掺杂水平(约 3%)提供了相稳定性和离子电导率之间的平衡。
更新日期:2024-11-29
中文翻译:

通过阴离子掺杂增强基于 LLZO 的固体电解质中的锂离子传导以实现高级储能:来自分子动力学模拟的见解
固态电解质 (SSE) 因其在储能应用中具有增强的安全性、更高的稳定性和能量密度,已成为传统液体电解质的有前途的替代品。在 SSE 中,立方锂7La3Zr2O12 (LLZO) 被认为特别有前途,具有高锂离子电导率、对金属阳极的高化学稳定性和较宽的电化学稳定性窗口。然而,在纯 LLZO 中冷却过程中,立方相会转化为导电性较低的四方相。掺杂是在较低温度下稳定立方 LLZO 并提高离子电导率的最有效方法之一。虽然对阳离子位点取代进行了广泛的研究,但对阴离子掺杂的研究非常有限。我们研究了氟掺杂对 LLZO 相稳定性和离子电导率的影响,使用基于可极化壳模型电位的分子动力学 (MD) 模拟,在 300-1400 K 的宽温度范围内探索了 1% 至 10% 的氟浓度。我们的结果表明,3% 氟掺杂在室温下达到最高的扩散系数 (3.69 × 10-7 cm2 s–1),而最低的活化能 (∼0.22 eV) 也出现在 3% 左右的掺杂下,这与实验观察结果非常吻合。发现 1% 的掺杂不足以稳定立方相,而高氟浓度 (>4%) 由于点缺陷 VLi 和 FO• 之间更强的静电相互作用而抑制了离子迁移途径。还计算了缺陷形成能量以研究缺陷形成和相互作用及其对锂离子传导的影响。 还通过使用 MD 模拟的轨迹来探索锂离子扩散途径和机制。这项研究为氟掺杂 LLZO 的优化提供了见解,表明适度的掺杂水平(约 3%)提供了相稳定性和离子电导率之间的平衡。






























 京公网安备 11010802027423号
京公网安备 11010802027423号