当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total synthesis of (–)-rhynchine A, (+)-rhynchine C and (+)-rhynchine E
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-30 , DOI: 10.1039/d4qo01987c Yinghao Cao, Xian Lu, Yuan-Yang Li, Lihua Huang, Beiling Gao
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-30 , DOI: 10.1039/d4qo01987c Yinghao Cao, Xian Lu, Yuan-Yang Li, Lihua Huang, Beiling Gao
|
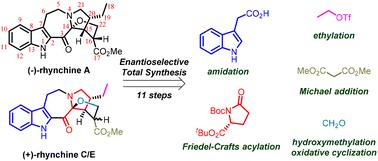
The first enantioselective total synthesis of (–)-rhynchine A, (+)-rhynchine C and (+)-rhynchine E has been successfully achieved in 11 steps via a convergent strategy. This efficient synthetic strategy provides 54 mg, 81 mg and 539 mg quantities of the above natural products at once, respectively, and revolves around three crucial transformations: firstly, substrate-controlled stereoselective ethylation and Michael addition are employed for the swift synthesis of the chiral proline fragment. Secondly, a mild Friedel–Crafts acylation is utilized for the formation of the tetracyclic skeleton. Lastly, a one-pot hydroxymethylation/oxidative cyclization method is employed for the construction of the tetrahydrofuran ring.
更新日期:2024-12-05






























 京公网安备 11010802027423号
京公网安备 11010802027423号