当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High selectivity of CO2 capture with single- and double-walled carbon nanotubes
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-29 , DOI: 10.1039/d4en00496e Winarto, Lilis Yuliati, Purnami, Paul E. Brumby, Kenji Yasuoka
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-29 , DOI: 10.1039/d4en00496e Winarto, Lilis Yuliati, Purnami, Paul E. Brumby, Kenji Yasuoka
|
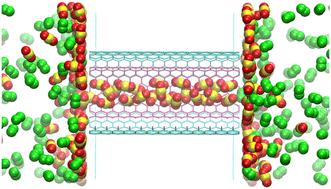
An excessive concentration of greenhouse gases, most significantly carbon dioxide (CO2), in the atmosphere has led to the serious environmental issue of global warming. Carbon capture is a suitable strategy to reduce the increase of CO2 in the atmosphere due to fossil fuel combustion. Innovative technologies for CO2 capture are urgently required and this is an area of intensive study in order to improve efficiency and reduce operational costs. In this work, we applied molecular dynamics simulations to demonstrate the ability of single-walled carbon nanotubes (SWCNT) and double-walled carbon nanotubes (DWCNT) to capture CO2 from flue gases. Both SWCNTs and DWCNTs prefer to adsorb CO2 rather than N2 and O2, resulting in a separation effect. CO2 molecules form a solid ice structure in the carbon nanotubes (CNT) while N2 and O2 remain gaseous. As a result, the potential energy of the CO2 structure inside the CNTs is lower than that of the N2 or O2 structures. This implies that CO2 is more stable in the CNTs. Therefore, the formation of these solid CO2 structures plays an important role in the process of capturing CO2via CNTs. Moreover, the van der Waals interactions between CO2 molecules and the CNT walls make a significant contribution to the separation of CO2 as well. The potential energy of the CO2–CNT wall interactions is significantly lower than those of N2–CNT wall or O2–CNT wall interactions. In addition, the presence of a second wall in DWCNTs causes even stronger attractive CO2–CNT wall van der Waals interactions than those found in SWCNTs. As a result, the CO2 capturing effect of DWCNT is greater than that of SWCNT.
中文翻译:

使用单壁和双壁碳纳米管进行 CO2 捕获的高选择性
大气中温室气体的过高浓度,尤其是二氧化碳 (CO2),导致了全球变暖的严重环境问题。碳捕获是减少化石燃料燃烧导致大气中 CO2 增加的合适策略。迫切需要创新的 CO2 捕获技术,这是一个需要深入研究的领域,以提高效率并降低运营成本。在这项工作中,我们应用分子动力学模拟来证明单壁碳纳米管 (SWCNT) 和双壁碳纳米管 (DWCNT) 从烟气中捕获CO2 的能力。SWCNT 和 DWCNT 都喜欢吸附 CO2 而不是 N2 和 O2,从而产生分离效果。CO2 分子在碳纳米管 (CNT) 中形成固体冰结构,而 N2 和 O2 保持气态。因此,碳纳米管内部 CO2 结构的势能低于 N2 或 O2 结构的势能。这意味着 CO2 在 CNT 中更稳定。因此,这些固体 CO2 结构的形成在通过 CNT 捕获 CO2 的过程中起着重要作用。此外,CO2 分子和 CNT 壁之间的范德华相互作用也对 CO2 的分离做出了重大贡献。 CO2-CNT 壁相互作用的势能明显低于 N2-CNT 壁或 O2-CNT 壁相互作用的势能。此外,DWCNT 中存在第二壁会导致比 SWCNT 中更强的有吸引力的 CO2-CNT 壁范德华相互作用。因此,DWCNT 的 CO2 捕获效果大于 SWCNT。
更新日期:2024-11-29
中文翻译:

使用单壁和双壁碳纳米管进行 CO2 捕获的高选择性
大气中温室气体的过高浓度,尤其是二氧化碳 (CO2),导致了全球变暖的严重环境问题。碳捕获是减少化石燃料燃烧导致大气中 CO2 增加的合适策略。迫切需要创新的 CO2 捕获技术,这是一个需要深入研究的领域,以提高效率并降低运营成本。在这项工作中,我们应用分子动力学模拟来证明单壁碳纳米管 (SWCNT) 和双壁碳纳米管 (DWCNT) 从烟气中捕获CO2 的能力。SWCNT 和 DWCNT 都喜欢吸附 CO2 而不是 N2 和 O2,从而产生分离效果。CO2 分子在碳纳米管 (CNT) 中形成固体冰结构,而 N2 和 O2 保持气态。因此,碳纳米管内部 CO2 结构的势能低于 N2 或 O2 结构的势能。这意味着 CO2 在 CNT 中更稳定。因此,这些固体 CO2 结构的形成在通过 CNT 捕获 CO2 的过程中起着重要作用。此外,CO2 分子和 CNT 壁之间的范德华相互作用也对 CO2 的分离做出了重大贡献。 CO2-CNT 壁相互作用的势能明显低于 N2-CNT 壁或 O2-CNT 壁相互作用的势能。此外,DWCNT 中存在第二壁会导致比 SWCNT 中更强的有吸引力的 CO2-CNT 壁范德华相互作用。因此,DWCNT 的 CO2 捕获效果大于 SWCNT。






























 京公网安备 11010802027423号
京公网安备 11010802027423号