当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
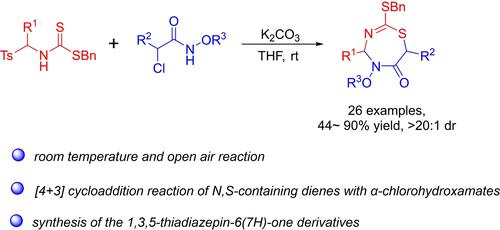
Base-Promoted [4+3] Cycloadditions of N-Thioacylimines with α-Chlorohydroxamates: Construction of 1,3,5-Thiadiazepin-6(7H)-One Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-27 , DOI: 10.1002/adsc.202401193 Yu-Di Liu, Yu-Heng Shao, Zhao-Lin He, Gang Wang
中文翻译:

α-氯异氧肟酸盐的 N-硫代酰亚胺的碱促进 [4+3] 环加成反应:1,3,5-硫二氮卓-6(7H)-酮衍生物的构建
更新日期:2024-11-27
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-27 , DOI: 10.1002/adsc.202401193 Yu-Di Liu, Yu-Heng Shao, Zhao-Lin He, Gang Wang
|
A metal-free base-promoted [4+3] cycloaddition reaction of N-thioacyl imines in situ generated from α-amido sulfones with α-chlorohydroxamates is described. The reaction allows the straightforward synthesis of 1,3,5-thiadiazepin-6(7H)-one derivatives in 44~90% yields. Moreover, a gram-scale reaction and a product transformation were conducted, which further increased the diversity of products.
中文翻译:

α-氯异氧肟酸盐的 N-硫代酰亚胺的碱促进 [4+3] 环加成反应:1,3,5-硫二氮卓-6(7H)-酮衍生物的构建
描述了由 α-酰胺基砜与 α-氯异氧肟酸盐原位生成的 N-硫酰基亚胺的无金属碱促进 [4+3] 环加成反应。该反应允许以 44~90% 的产率直接合成 1,3,5-噻二氮卓-6(7H)-酮衍生物。此外,还进行了克级反应和产物转化,进一步增加了产物的多样性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号