当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization and Scale-Up Synthesis of a Lappaconitine Alkaloid Derivative, QG3030, as a Novel Osteoanabolic Agent
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-29 , DOI: 10.1021/acs.oprd.4c00344 Heung Mo Kang, Chang Sang Moon, Yunchan Nam, Jiwoong Lim, Jiewan Kim, Tae-Hee Lee, Junho Lee, Mun Seog Chang, Jae Yeol Lee
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-29 , DOI: 10.1021/acs.oprd.4c00344 Heung Mo Kang, Chang Sang Moon, Yunchan Nam, Jiwoong Lim, Jiewan Kim, Tae-Hee Lee, Junho Lee, Mun Seog Chang, Jae Yeol Lee
|
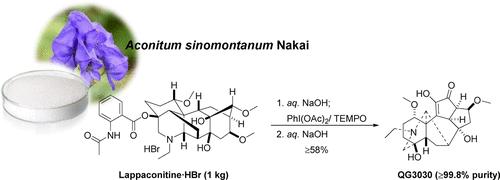
Our previous work revealed that the novel lappaconitine alkaloid derivative, QG3030 (6), has an enhanced osteogenesis effect in the ovariectomized rat model without acute oral toxicity. QG3030 (6) recently received approval for the Investigational New Drug application for its osteoporosis treatment from the Korean Ministry of Food and Drug Safety. Therefore, the need for an economical, large-scale production of QG3030 (6) motivated the development of a novel synthetic procedure for its clinical studies. We herein report an efficient, safe, and cost-effective synthesis of QG3030 (6) as a clinical candidate for osteoporosis treatment. As an optimized synthetic procedure, the reaction of lappaconitine·HBr (1·HBr, 1.0 kg scale) with co-oxidizing agents PhI(OAc)2-TEMPO (1.5 and 2 equiv) as a key step in a mixed EtOAc-acetone solution (v/v = 2/1) furnished α,β-unsaturated ketone (4), which was then treated with aq. NaOH to provide pure QG3030 (6, 352 g) in 58% overall yield with a purity of 99.8% after crystallization from EtOH–CH2Cl2. This pilot synthetic procedure was performed three times, and the reproducible results were obtained with both nearly identical yields and purities.
中文翻译:

Lappaconitine 生物碱衍生物 QG3030 作为新型骨合成代谢剂的优化和放大合成
我们之前的工作揭示了新型 lappaconitine 生物碱衍生物 QG3030 (6) 在去卵巢大鼠模型中具有增强的成骨作用,而没有急性口服毒性。QG3030 (6) 最近获得了韩国食品药品安全部批准用于治疗骨质疏松症的新药临床试验申请。因此,对 QG3030 的经济、大规模生产的需求 (6) 促使开发一种用于其临床研究的新型合成程序。我们在此报道了 QG3030 (6) 作为骨质疏松症治疗的临床候选药物的高效、安全且具有成本效益的合成。作为一种优化的合成工艺,lappaconitine·HBr (1·HBr,1.0 kg 刻度)与共氧化剂 PhI(OAc)2-TEMPO(1.5 和 2 当量)作为混合 EtOAc-丙酮溶液 (v/v = 2/1) 中的关键步骤,提供 α,β-不饱和酮 (4),然后用水处理。NaOH 提供纯 QG3030 (6, 352 g),总产率为 58%,从 EtOH–CH2Cl2 结晶后纯度为 99.8%。该中试合成程序进行了 3 次,以几乎相同的产量和纯度获得了可重现的结果。
更新日期:2024-11-29
中文翻译:

Lappaconitine 生物碱衍生物 QG3030 作为新型骨合成代谢剂的优化和放大合成
我们之前的工作揭示了新型 lappaconitine 生物碱衍生物 QG3030 (6) 在去卵巢大鼠模型中具有增强的成骨作用,而没有急性口服毒性。QG3030 (6) 最近获得了韩国食品药品安全部批准用于治疗骨质疏松症的新药临床试验申请。因此,对 QG3030 的经济、大规模生产的需求 (6) 促使开发一种用于其临床研究的新型合成程序。我们在此报道了 QG3030 (6) 作为骨质疏松症治疗的临床候选药物的高效、安全且具有成本效益的合成。作为一种优化的合成工艺,lappaconitine·HBr (1·HBr,1.0 kg 刻度)与共氧化剂 PhI(OAc)2-TEMPO(1.5 和 2 当量)作为混合 EtOAc-丙酮溶液 (v/v = 2/1) 中的关键步骤,提供 α,β-不饱和酮 (4),然后用水处理。NaOH 提供纯 QG3030 (6, 352 g),总产率为 58%,从 EtOH–CH2Cl2 结晶后纯度为 99.8%。该中试合成程序进行了 3 次,以几乎相同的产量和纯度获得了可重现的结果。






























 京公网安备 11010802027423号
京公网安备 11010802027423号