当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-boosted fluorinated prodrug hybrid nanoassemblies for bidirectional amplification of breast cancer ferroptosis
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-29 , DOI: 10.1016/j.jconrel.2024.11.053 Dongqi Sun, Xinxin Sun, Jianbin Shi, Xianbao Shi, Jin Sun, Cong Luo, Zhonggui He, Shenwu Zhang
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-29 , DOI: 10.1016/j.jconrel.2024.11.053 Dongqi Sun, Xinxin Sun, Jianbin Shi, Xianbao Shi, Jin Sun, Cong Luo, Zhonggui He, Shenwu Zhang
|
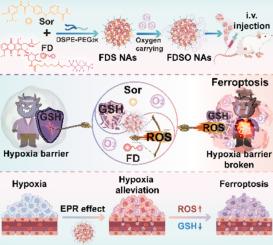
Ferroptosis, a novel form of cell death, has emerged as a promising approach in cancer therapy. However, the single ferroptosis inducer was ineffective, and the induction of ferroptosis was severely limited by hypoxia niches in breast cancer. Herein, we develop a disulfide bond-bridging fluorinated doxorubicin (DOX) prodrug, which can facilitate the formation of hybrid nanoassemblies (NAs) with sorafenib (Sor) through a molecular co-assembly strategy. The incorporation of fluorinated side chains enhances the oxygen-carrying capacity of the NAs, successfully reversing the redox offensive and defensive situation caused by the dilemma of hypoxia. The reactive oxygen species (ROS) generation capacity of DOX via nicotinamide adenine dinucleotide oxidase (NOXs) within hypoxic tumors is significantly enhanced due to the presence of fluorinated oxygen-carrying as a catalytic substrate. Furthermore, the depletion of nicotinamide adenine dinucleotide phosphate (NADPH) significantly impairs the synthesis of glutathione (GSH), which collaboratively inhibits GSH production with Sor. As expected, the NAs with bidirectional amplification of ROS production and GSH inhibition displays potent antitumor activity in 4 T1 breast cancer-bearing mice. Together, this study presents a novel nanotherapeutic approach for ferroptosis-driven tumor therapy.
中文翻译:

氧增强氟化前药杂交纳米组装体用于乳腺癌铁死亡的双向扩增
铁死亡是一种新型的细胞死亡,已成为癌症治疗中一种很有前途的方法。然而,单一铁死亡诱导剂无效,并且铁死亡的诱导受到乳腺癌中缺氧生态位的严重限制。在此,我们开发了一种二硫键桥接氟化多柔比星 (DOX) 前药,它可以通过分子共组装策略促进与索拉非尼 (Sor) 形成杂化纳米组装 (NA)。氟化侧链的掺入增强了 NA 的携氧能力,成功扭转了缺氧困境造成的氧化还原攻守局面。由于存在氟化氧携带氧作为催化底物,DOX 通过烟酰胺腺嘌呤二核苷酸氧化酶 (NOX) 在缺氧肿瘤内生成活性氧 (ROS) 的能力显着增强。此外,烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 的耗竭显着损害谷胱甘肽 (GSH) 的合成,谷胱甘肽与 Sor 共同抑制 GSH 的产生。正如预期的那样,ROS 产生和 GSH 抑制双向扩增的 NAs 在 4 只 T1 荷癌小鼠中显示出有效的抗肿瘤活性。总之,本研究提出了一种用于铁死亡驱动的肿瘤治疗的新型纳米治疗方法。
更新日期:2024-11-29
中文翻译:

氧增强氟化前药杂交纳米组装体用于乳腺癌铁死亡的双向扩增
铁死亡是一种新型的细胞死亡,已成为癌症治疗中一种很有前途的方法。然而,单一铁死亡诱导剂无效,并且铁死亡的诱导受到乳腺癌中缺氧生态位的严重限制。在此,我们开发了一种二硫键桥接氟化多柔比星 (DOX) 前药,它可以通过分子共组装策略促进与索拉非尼 (Sor) 形成杂化纳米组装 (NA)。氟化侧链的掺入增强了 NA 的携氧能力,成功扭转了缺氧困境造成的氧化还原攻守局面。由于存在氟化氧携带氧作为催化底物,DOX 通过烟酰胺腺嘌呤二核苷酸氧化酶 (NOX) 在缺氧肿瘤内生成活性氧 (ROS) 的能力显着增强。此外,烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 的耗竭显着损害谷胱甘肽 (GSH) 的合成,谷胱甘肽与 Sor 共同抑制 GSH 的产生。正如预期的那样,ROS 产生和 GSH 抑制双向扩增的 NAs 在 4 只 T1 荷癌小鼠中显示出有效的抗肿瘤活性。总之,本研究提出了一种用于铁死亡驱动的肿瘤治疗的新型纳米治疗方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号