当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-responsive hydrogel with gambogic acid and calcium nanowires for promoting mitochondrial apoptosis in osteosarcoma
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-29 , DOI: 10.1016/j.jconrel.2024.11.055 Lei Yang, Qiang Sun, Shiyin Chen, Dongshen Ma, Yao Qi, Hongmei Liu, Sumin Tan, Qin Yue, Lulu Cai
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-29 , DOI: 10.1016/j.jconrel.2024.11.055 Lei Yang, Qiang Sun, Shiyin Chen, Dongshen Ma, Yao Qi, Hongmei Liu, Sumin Tan, Qin Yue, Lulu Cai
|
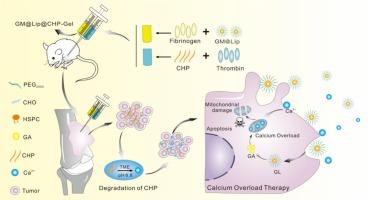
Calcium (Ca2+ ) overload therapy gained significant attention in oncology. However, its therapeutic efficacy remained limited due to insufficient Ca2+ accumulation at the tumor site and suboptimal intracellular Ca2+ influx. In this study, gambogic acid (GA), a natural phenolic compound known to promote Ca2+ influx, was encapsulated within an enzyme-triggered, pH-responsive hydrogel (GM@Lip@CHP-Gel) containing Ca2+ hydrogen phosphate nanowires (CHP) to achieve a synergistic approach for bone tumor therapy. GM@Lip@CHP-Gel selectively responded to the slightly acidic tumor microenvironment, triggering degradation of its 3D network structure and sustaining the release of GA and Ca2+ into tumor cells. GA subsequently stimulated Ca2+ influx in tumor cells, effectively disrupting Ca2+ homeostasis. CHP nanowires served as a continuous Ca2+ source, enhancing GA-mediated Ca2+ overload and promoting mitochondrial apoptosis in tumor cells. The combined strategy resulted in an in vivo tumor suppression rate of 79 % and a lung metastasis inhibition rate of 89.4 %, with a protective effect on bone tissue. The naturally derived, Ca2+ -mediated treatment demonstrated physiochemical stability in physiological environments and minimized side effects on healthy organs, positioning it as a promising approach for clinical bone cancer therapy.
中文翻译:

含藤黄酸和钙纳米线的 pH 响应性水凝胶促进骨肉瘤线粒体凋亡
钙 (Ca2+) 超负荷治疗在肿瘤学中受到广泛关注。然而,由于肿瘤部位 Ca2+ 积累不足和细胞内 Ca2+ 内流不理想,其治疗效果仍然有限。在这项研究中,藤黄酸 (GA) 是一种已知可促进 Ca2+ 内流的天然酚类化合物,被封装在含有 Ca2+ 磷酸氢纳米线 (CHP) 的酶触发、pH 响应性水凝胶 (GM@Lip@CHP-Gel) 中,以实现骨肿瘤治疗的协同方法。GM@Lip@CHP-Gel 选择性地响应微酸性肿瘤微环境,触发其 3D 网络结构的降解并维持 GA 和 Ca2+ 释放到肿瘤细胞中。GA 随后刺激肿瘤细胞中的 Ca2+ 内流,有效破坏 Ca2+ 稳态。CHP 纳米线作为连续的 Ca2 + 来源,增强 GA 介导的 Ca2 + 过载并促进肿瘤细胞中的线粒体凋亡。联合策略导致体内肿瘤抑制率为 79 %,肺转移抑制率为 89.4 %,对骨组织具有保护作用。这种天然来源的 Ca2+ 介导的治疗在生理环境中表现出理化稳定性,并最大限度地减少了对健康器官的副作用,使其成为临床骨癌治疗的有前途的方法。
更新日期:2024-11-29
中文翻译:

含藤黄酸和钙纳米线的 pH 响应性水凝胶促进骨肉瘤线粒体凋亡
钙 (Ca2+) 超负荷治疗在肿瘤学中受到广泛关注。然而,由于肿瘤部位 Ca2+ 积累不足和细胞内 Ca2+ 内流不理想,其治疗效果仍然有限。在这项研究中,藤黄酸 (GA) 是一种已知可促进 Ca2+ 内流的天然酚类化合物,被封装在含有 Ca2+ 磷酸氢纳米线 (CHP) 的酶触发、pH 响应性水凝胶 (GM@Lip@CHP-Gel) 中,以实现骨肿瘤治疗的协同方法。GM@Lip@CHP-Gel 选择性地响应微酸性肿瘤微环境,触发其 3D 网络结构的降解并维持 GA 和 Ca2+ 释放到肿瘤细胞中。GA 随后刺激肿瘤细胞中的 Ca2+ 内流,有效破坏 Ca2+ 稳态。CHP 纳米线作为连续的 Ca2 + 来源,增强 GA 介导的 Ca2 + 过载并促进肿瘤细胞中的线粒体凋亡。联合策略导致体内肿瘤抑制率为 79 %,肺转移抑制率为 89.4 %,对骨组织具有保护作用。这种天然来源的 Ca2+ 介导的治疗在生理环境中表现出理化稳定性,并最大限度地减少了对健康器官的副作用,使其成为临床骨癌治疗的有前途的方法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号