当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Imidazolium-Based Ionic Liquid Electrolytes for Fluoride Ion Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-11-27 , DOI: 10.1021/acsenergylett.4c02663 Omar Alshangiti, Giulia Galatolo, Camilla Di Mino, Thomas F. Headen, Jacob Christianson, Simone Merotto, Gregory J. Rees, Yoan Delavoux, Małgorzata Swadźba-Kwaśny, Mauro Pasta
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-11-27 , DOI: 10.1021/acsenergylett.4c02663 Omar Alshangiti, Giulia Galatolo, Camilla Di Mino, Thomas F. Headen, Jacob Christianson, Simone Merotto, Gregory J. Rees, Yoan Delavoux, Małgorzata Swadźba-Kwaśny, Mauro Pasta
|
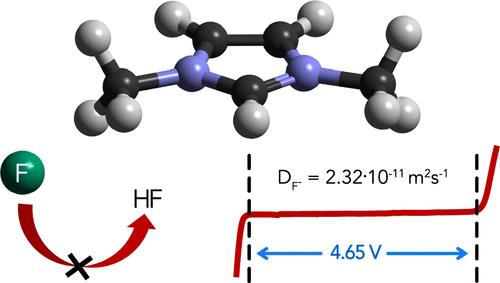
The fluoride-ion battery (FIB) is a post-lithium anionic battery that utilizes the fluoride-ion shuttle, achieving high theoretical energy densities of up to 1393 Wh L–1 without relying on critical minerals. However, developing liquid electrolytes for FIBs has proven arduous due to the low solubility of fluoride salts and the chemical reactivity of the fluoride ion. By introducing a chemically stable electrolyte based on 1,3-dimethylimidazolium [MMIm] bis(trifluoromethanesulfonyl)imide [TFSI] and tetramethylammonium fluoride (TMAF), we achieve an electrochemical stability window (ESW) of 4.65 V, ionic conductivity of 9.53 mS cm–1, and a solubility of 0.67 m. The origin of this high solubility and the solvation structure were investigated using NMR spectroscopy and neutron total scattering, showing a fluoride solvation driven by strong electrostatic interactions and weak hydrogen bonding without covalent H–F character. This indicates the chemical stability of 1,3-dimethylimidazolium toward the fluoride ion and its potential as an electrolyte for high-voltage FIBs.
中文翻译:

用于氟离子电池的咪唑基离子液体电解质
氟离子电池 (FIB) 是一种后锂离子电池,它利用氟离子穿梭机,在不依赖关键矿物的情况下实现高达 1393 Wh L–1 的高理论能量密度。然而,由于氟化物盐的低溶解度和氟离子的化学反应性,为 FIB 开发液体电解质已被证明是艰巨的。通过引入基于 1,3-二甲基咪唑 [MMIm] 双(三氟甲磺酰基)酰亚胺 [TFSI] 和四甲基氟化铵 (TMAF) 的化学稳定电解质,我们实现了 4.65 V 的电化学稳定性窗口 (ESW)、9.53 mS cm–1 的离子电导率和 0.67 m 的溶解度。使用 NMR 波谱和中子全散射研究了这种高溶解度的来源和溶剂化结构,显示了由强静电相互作用和弱氢键驱动的氟化物溶剂化,没有共价 H-F 特性。这表明 1,3-二甲基咪唑对氟离子的化学稳定性及其作为高压 FIB 电解质的潜力。
更新日期:2024-11-27
中文翻译:

用于氟离子电池的咪唑基离子液体电解质
氟离子电池 (FIB) 是一种后锂离子电池,它利用氟离子穿梭机,在不依赖关键矿物的情况下实现高达 1393 Wh L–1 的高理论能量密度。然而,由于氟化物盐的低溶解度和氟离子的化学反应性,为 FIB 开发液体电解质已被证明是艰巨的。通过引入基于 1,3-二甲基咪唑 [MMIm] 双(三氟甲磺酰基)酰亚胺 [TFSI] 和四甲基氟化铵 (TMAF) 的化学稳定电解质,我们实现了 4.65 V 的电化学稳定性窗口 (ESW)、9.53 mS cm–1 的离子电导率和 0.67 m 的溶解度。使用 NMR 波谱和中子全散射研究了这种高溶解度的来源和溶剂化结构,显示了由强静电相互作用和弱氢键驱动的氟化物溶剂化,没有共价 H-F 特性。这表明 1,3-二甲基咪唑对氟离子的化学稳定性及其作为高压 FIB 电解质的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号