当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote C(sp3)–H acylation of amides under photoredox cooperative N-heterocyclic carbene/palladium catalysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-29 , DOI: 10.1039/d4qo01785d Xiaoyu Lin, Haoran Huang, Fanyuanhang Yang, Yuxi Ren, Yuzhen Gao, Weiping Su
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-29 , DOI: 10.1039/d4qo01785d Xiaoyu Lin, Haoran Huang, Fanyuanhang Yang, Yuxi Ren, Yuzhen Gao, Weiping Su
|
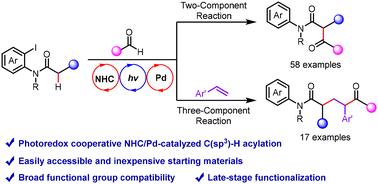
Direct acylation of amides at C(sp3)–H sites from aldehydes is one of the most convenient methods to access highly valuable keto-amides; yet it remains challenging and less developed. Herein, we report a photoredox cooperative NHC/Pd-catalyzed method that enables C(sp3)–H acylation for the quick construction of keto-amide derivatives from readily available amides and aldehydes. This work demonstrates the generation of ketyl radicals from aldehydes under NHC/Pd catalysis and alkyl radicals via 1,5-hydrogen atom transfer from aryl radicals under photoredox/Pd catalysis, thus providing two- and three-component reactions for the synthesis of α- and γ-keto-amides through radical–radical coupling reactions. This work shows good compatibility with a large variety of functional groups, as demonstrated by more than 75 examples, including complex bioactive compounds. Mechanistic studies including several control reactions shed light on the mechanism of this photoredox cooperative NHC/Pd-catalyzed reaction.
中文翻译:

光氧化还原协同 N-杂环卡宾/钯催化下酰胺的远程 C(sp3)–H 酰化
醛在 C(sp3)-H 位点的酰胺直接酰化是获得高价值酮酰胺的最便捷方法之一;然而,它仍然具有挑战性且欠发达。在此,我们报道了一种光氧化还原协同 NHC/Pd 催化方法,该方法能够实现 C(sp3)-H 酰化,以便从现成的酰胺和醛中快速构建酮酰胺衍生物。本工作证明了在 NHC/Pd 催化下醛生成酮基自由基,在光氧化还原/Pd 催化下通过芳基自由基通过 1,5-氢原子转移生成烷基自由基,从而为通过自由基-自由基偶联反应合成 α 和 γ-酮基酰胺提供双组分和三组分反应。这项工作显示出与多种官能团的良好相容性,包括复杂的生物活性化合物在内的超过 75 个例子证明了这一点。包括几个对照反应在内的机理研究阐明了这种光氧化还原协同 NHC/Pd 催化反应的机理。
更新日期:2024-11-29
中文翻译:

光氧化还原协同 N-杂环卡宾/钯催化下酰胺的远程 C(sp3)–H 酰化
醛在 C(sp3)-H 位点的酰胺直接酰化是获得高价值酮酰胺的最便捷方法之一;然而,它仍然具有挑战性且欠发达。在此,我们报道了一种光氧化还原协同 NHC/Pd 催化方法,该方法能够实现 C(sp3)-H 酰化,以便从现成的酰胺和醛中快速构建酮酰胺衍生物。本工作证明了在 NHC/Pd 催化下醛生成酮基自由基,在光氧化还原/Pd 催化下通过芳基自由基通过 1,5-氢原子转移生成烷基自由基,从而为通过自由基-自由基偶联反应合成 α 和 γ-酮基酰胺提供双组分和三组分反应。这项工作显示出与多种官能团的良好相容性,包括复杂的生物活性化合物在内的超过 75 个例子证明了这一点。包括几个对照反应在内的机理研究阐明了这种光氧化还原协同 NHC/Pd 催化反应的机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号