Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2 production from the photocatalytic reforming of ethylene glycol: Effect of TiO2 crystalline phase on photo-oxidation mechanism
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-28 , DOI: 10.1016/j.jcat.2024.115876 Luke Roebuck, Helen Daly, Lan Lan, Joseph Parker, Angus Gostick, Nathan Skillen, Sarah J. Haigh, Marta Falkowska, Christopher Hardacre
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-28 , DOI: 10.1016/j.jcat.2024.115876 Luke Roebuck, Helen Daly, Lan Lan, Joseph Parker, Angus Gostick, Nathan Skillen, Sarah J. Haigh, Marta Falkowska, Christopher Hardacre
|
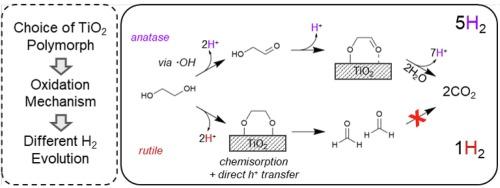
In this work, the mechanism of H2 production from ethylene glycol as a model compound for photoreforming over platinized TiO2 is presented with particular focus on the effect of the TiO2 polymorph. It was found that Pt/anatase and Pt/anatase:rutile (P25) had similar H2 production activities and both catalysts followed an indirect oxidation pathway where ethylene glycol was oxidised via hydroxyl radicals to glycolaldehyde. In contrast, Pt/rutile primarily oxidised ethylene glycol directly into formaldehyde. The formaldehyde was unable to react further, which significantly reduced the formation of hydrogen despite similar conversion of ethylene glycol compared with the other supports used. We propose that these differences are due to different adsorption behaviour and hole transfer mechanism on the different TiO2 crystalline phases. In particular, ethylene glycol complexation on Ti5c sites on the dominant (110) facet of rutile leads to a direct hole transfer and an oxidative C–C cleavage mechanism prevailing.
中文翻译:

乙二醇光催化重整制 H2:TiO2 晶相对光氧化机理的影响
在这项工作中,提出了乙二醇产生 H2 的机制,作为铂化 TiO2 光重整的模型化合物,特别关注 TiO2 多晶型物的影响。研究发现,Pt/锐钛矿和 Pt/锐钛矿:金红石 (P25) 具有相似的 H2 生产活性,并且两种催化剂都遵循间接氧化途径,其中乙二醇通过羟基自由基氧化成乙二醇醛。相比之下,Pt/金红石主要将乙二醇直接氧化成甲醛。甲醛无法进一步反应,尽管与使用的其他载体相比,乙二醇的转化率相似,但氢气的形成显着减少。我们认为,这些差异是由于不同 TiO2 晶相上的不同吸附行为和空穴转移机制造成的。特别是,金红石主要 (110) 面上 Ti5c 位点上的乙二醇络合导致直接空穴转移和氧化 C-C 裂解机制。
更新日期:2024-11-28
中文翻译:

乙二醇光催化重整制 H2:TiO2 晶相对光氧化机理的影响
在这项工作中,提出了乙二醇产生 H2 的机制,作为铂化 TiO2 光重整的模型化合物,特别关注 TiO2 多晶型物的影响。研究发现,Pt/锐钛矿和 Pt/锐钛矿:金红石 (P25) 具有相似的 H2 生产活性,并且两种催化剂都遵循间接氧化途径,其中乙二醇通过羟基自由基氧化成乙二醇醛。相比之下,Pt/金红石主要将乙二醇直接氧化成甲醛。甲醛无法进一步反应,尽管与使用的其他载体相比,乙二醇的转化率相似,但氢气的形成显着减少。我们认为,这些差异是由于不同 TiO2 晶相上的不同吸附行为和空穴转移机制造成的。特别是,金红石主要 (110) 面上 Ti5c 位点上的乙二醇络合导致直接空穴转移和氧化 C-C 裂解机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号