Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed cyanation of C—S bond using CO2 and NH3
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-28 , DOI: 10.1016/j.jcat.2024.115870 Qin Shi, Yang Li, Yudong Li, Yanan Dong, Shaoli Liu, Zhen Li, Lin He, Liwei Sun, Yuehui Li
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-28 , DOI: 10.1016/j.jcat.2024.115870 Qin Shi, Yang Li, Yudong Li, Yanan Dong, Shaoli Liu, Zhen Li, Lin He, Liwei Sun, Yuehui Li
|
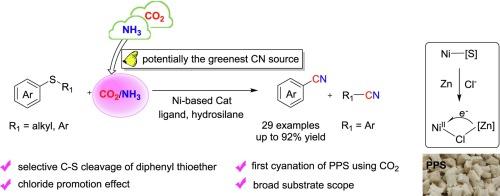
Catalytic cleavage of C—S bonds is an important approach in the construction of functionalized molecules. Herein, we developed the catalytic synthesis of nitriles from thioethers, utilizing CO2 as the carbon source for the first time. This work addressed the challenge of avoiding toxic cyanides by generating the “CN” group via isocyanate intermediates from CO2 and NH3 with the aid of Ni-phosphine catalysts and hydrosilane. A wide range of aryl thioethers were efficiently converted into the corresponding aryl nitriles, demonstrating good functional groups tolerance including fluorine, morpholine, piperazine amide, and cyano group. Additionally, chloride anion played essential roles probably through facilitating the generation of Ni (I) species by promoting Ni—S bond cleavage, thus enhancing the overall reaction efficiency.
中文翻译:

使用 CO2 和 NH3 的镍催化 C-S 键氰化反应
C-S 键的催化裂解是构建功能化分子的重要方法。在此,我们首次利用 CO2 作为碳源,开发了从硫醚催化合成腈的方法。这项工作在镍膦催化剂和氢硅烷的帮助下,通过 CO2 和 NH3 的异氰酸酯中间体生成“CN”基团,解决了避免有毒氰化物的挑战。多种芳基硫醚被有效地转化为相应的芳基腈,表现出良好的官能团耐受性,包括氟、吗啉、哌嗪酰胺和氰基。此外,氯阴离子可能通过促进 Ni-S 键裂解来促进 Ni (I) 物质的生成,从而提高整体反应效率,从而发挥了重要作用。
更新日期:2024-11-28
中文翻译:

使用 CO2 和 NH3 的镍催化 C-S 键氰化反应
C-S 键的催化裂解是构建功能化分子的重要方法。在此,我们首次利用 CO2 作为碳源,开发了从硫醚催化合成腈的方法。这项工作在镍膦催化剂和氢硅烷的帮助下,通过 CO2 和 NH3 的异氰酸酯中间体生成“CN”基团,解决了避免有毒氰化物的挑战。多种芳基硫醚被有效地转化为相应的芳基腈,表现出良好的官能团耐受性,包括氟、吗啉、哌嗪酰胺和氰基。此外,氯阴离子可能通过促进 Ni-S 键裂解来促进 Ni (I) 物质的生成,从而提高整体反应效率,从而发挥了重要作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号