当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic haloallylation/Zr-mediated dienyne cyclization/isomerization sequence for tailored cyclopentadiene substitution
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-27 , DOI: 10.1039/d4qo02020k Marko Gobin, Ivana Nikšić-Franjić, Nikola Topolovčan
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-27 , DOI: 10.1039/d4qo02020k Marko Gobin, Ivana Nikšić-Franjić, Nikola Topolovčan
|
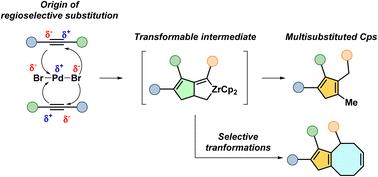
The chemical properties and reactivity of cyclopentadienes (Cp) originate from the number and nature of attached functionalities. Even a slight change in their molecular architecture dramatically affects their application in organic synthesis and the performance of the respective Cp complexes in catalytic transformations. Thus, the current demand for multisubstituted cyclopentadienes requires a strategic design, allowing substituents to be installed around the Cp ring to fine-tune its reactivity profile. Herein, we present a five-step synthetic sequence that allows site-selective positioning of diverse functional groups that are otherwise difficult to attach with current methods. A judicious choice of stereoelectronically defined internal alkynes enabled regioselective bromoallylation, resulting in 1-bromo-1,4-dienes bearing three functionalities that will be part of the target Cp. Continued substitution-enrichment through the Sonogashira coupling firstly gave ornamented dienynes that upon Zr-mediated cyclization afforded a series of cyclopentenes. Finally, an acid-catalyzed exo-to-endo double bond isomerization concluded the controlled allocation of functionalities and gave a series of tetrasubstituted cyclopentadienes. Additionally, the transformability of the organozirconium intermediate enables the synthesis of bicyclic cyclopentadienes.
中文翻译:

催化卤烯丙基化/Zr 介导的二炔环化/异构化序列,用于定制的环戊二烯取代
环戊二烯 (Cp) 的化学性质和反应性源于连接官能团的数量和性质。即使它们的分子结构发生微小的变化,也会极大地影响它们在有机合成中的应用以及相应 Cp 配合物在催化转化中的性能。因此,当前对多取代环戊二烯的需求需要一个战略性设计,允许在 Cp 环周围安装取代基以微调其反应性曲线。在此,我们提出了一个五步合成序列,该序列允许对不同的官能团进行位点选择性定位,否则这些官能团很难用当前方法连接。明智地选择立体电子定义的内部炔烃实现了区域选择性溴烯丙基化,导致 1-溴-1,4-二烯具有三种官能团,它们将成为目标 Cp 的一部分。通过 Sonogashira 偶联的持续取代富集首先得到了装饰性的二炔烯,在 Zr 介导的环化作用下,产生了一系列环戊烯。最后,酸催化的 exo 到 endo 双键异构化结束了官能团的受控分配,并给出了一系列四取代的环戊二烯。此外,有机锆中间体的可转化性使双环戊二烯的合成成为可能。
更新日期:2024-12-02
中文翻译:

催化卤烯丙基化/Zr 介导的二炔环化/异构化序列,用于定制的环戊二烯取代
环戊二烯 (Cp) 的化学性质和反应性源于连接官能团的数量和性质。即使它们的分子结构发生微小的变化,也会极大地影响它们在有机合成中的应用以及相应 Cp 配合物在催化转化中的性能。因此,当前对多取代环戊二烯的需求需要一个战略性设计,允许在 Cp 环周围安装取代基以微调其反应性曲线。在此,我们提出了一个五步合成序列,该序列允许对不同的官能团进行位点选择性定位,否则这些官能团很难用当前方法连接。明智地选择立体电子定义的内部炔烃实现了区域选择性溴烯丙基化,导致 1-溴-1,4-二烯具有三种官能团,它们将成为目标 Cp 的一部分。通过 Sonogashira 偶联的持续取代富集首先得到了装饰性的二炔烯,在 Zr 介导的环化作用下,产生了一系列环戊烯。最后,酸催化的 exo 到 endo 双键异构化结束了官能团的受控分配,并给出了一系列四取代的环戊二烯。此外,有机锆中间体的可转化性使双环戊二烯的合成成为可能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号