当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pivotal role of solid phase interactions in the pressure-induced bi-stability of cyanide-bridged Fe2Co2 square complexes
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2024-11-27 , DOI: 10.1039/d4qi02499k Buqin Xu, Yanling Li, Geoffrey Gontard, Keevin Béneut, Paraskevas Parisiades, Maxime Deutsch, Rodrigue Lescouëzec
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2024-11-27 , DOI: 10.1039/d4qi02499k Buqin Xu, Yanling Li, Geoffrey Gontard, Keevin Béneut, Paraskevas Parisiades, Maxime Deutsch, Rodrigue Lescouëzec
|
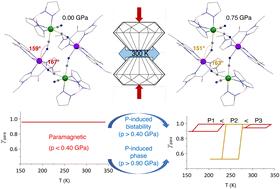
Cyanide-bridged FeCo coordination clusters, recognized as exceptional candidates for molecular switches, have been the subject of extensive research efforts aimed at unraveling the key factors governing the charge transfer process. Previously, we have observed that the square complex {[Fe(Tp)(CN)3]2[Co(vbik)2]2}2+ with Tp = tris(pyrazolyl)borate and vbik = bis(1-vinyl-2-imidazolyl)ketone, abbreviated as {Fe2Co2}, undergoes thermal charge transfer in MeOH solution near room temperature allowing the obtention of a solvatomorph pair, {FeIII2CoII2}·2BF4·2MeOH (1) and {FeII2CoIII2}·2BF4·10H2O·2MeOH (2), exhibiting distinct electronic configurations at 300 K. While 2 maintains its charge transfer ability in the solid state, 1 is trapped in the paramagnetic state by solid phase interactions down to 2 K, which makes it a good candidate for investigating electron transfer under hydrostatic pressure, an external stimulus scarcely used for these systems. In the present work, we demonstrated that the synthesis method can be used to obtain a new solvatomorph with remarkable pressure-induced electron transfer. The paramagnetic {FeIII2CoII2}·2(PF6)·2MeOH (3) and the diamagnetic {FeII2CoIII2}·2(PF6)·nH2O·mMeOH (4), isostructural to 1 and 2, respectively, were obtained. The stronger intermolecular interactions due to the PF6 anion lead to a greater distortion of the core structures of 3 and 4, affecting their magnetic properties under ambient and hydrostatic pressure. Notably, 3 exhibits a partial pressure-induced conversion from a paramagnetic to a diamagnetic state followed by a back conversion above a pressure threshold value, which is rationalized by a symmetry-breaking phase transition at ca. 0.96–1.0 GPa. This unusual behavior has been analyzed using magnetometry, X-ray diffraction, and μ-Raman spectroscopy under various pressures. A deeper understanding of the electron transfer process in 3 was achieved by analyzing its structural data under various pressures and comparing them to those of 1. The distinct electron transfer behaviors observed in the two complexes are likely correlated to the differing distortions in their square core structures induced by pressure.
中文翻译:

固相相互作用在氰化物桥式 Fe2Co2 方形配合物压力诱导的双稳定性中的关键作用
氰化物桥式 FeCo 配位簇被认为是分子开关的特殊候选者,一直是旨在揭示控制电荷转移过程的关键因素的广泛研究工作的主题。以前,我们已经观察到,具有 Tp = 三(吡唑基)硼酸盐和 vbik = 双(1-乙烯基-2-咪唑基)酮的方配合物 {[Fe(Tp)(CN)3]2[Co(vbik)2]2+,缩写为 {Fe2Co2},在接近室温的 MeOH 溶液中发生热电荷转移,从而获得溶剂晶型对,{FeIII2CoII2}·2BF4·2MeOH (1) 和 {FeII2CoIII2}·2BF4·10H2O·2MeOH (2),在 300 K 时表现出不同的电子构型。虽然 2 在固态下保持其电荷转移能力,但 1 在顺磁状态中被低至 2 K 的固相相互作用捕获,这使其成为研究静水压力下电子转移的良好候选者,静水压力是这些系统很少使用的外部刺激。在本工作中,我们证明了合成方法可用于获得具有显着压力诱导电子转移的新型溶剂晶型。 顺磁性 {FeIII2CoII2}·2(PF6)·2MeOH (3) 和抗磁性 {FeII2CoIII2}·2(PF6)·nH2O·米获得了 MeOH (4),分别与 1 和 2 同构。由于 PF6 阴离子引起的更强分子间相互作用导致 3 和 4 的核心结构发生更大的变形,从而影响它们在环境和静水压力下的磁性。值得注意的是,3 表现出从顺磁状态到抗磁状态的分压诱导转换,然后是高于压力阈值的反向转换,这可以通过大约 0.96–1.0 GPa 的对称性打破相变来合理化。在各种压力下,已经使用磁力计、X 射线衍射和 μ-Raman 光谱分析了这种不寻常的行为。通过分析其在各种压力下的结构数据并将其与 1 的结构数据进行比较,可以更深入地了解 3 中的电子转移过程。在两种配合物中观察到的不同电子转移行为可能与压力引起的方形核心结构的不同扭曲有关。
更新日期:2024-11-27
中文翻译:

固相相互作用在氰化物桥式 Fe2Co2 方形配合物压力诱导的双稳定性中的关键作用
氰化物桥式 FeCo 配位簇被认为是分子开关的特殊候选者,一直是旨在揭示控制电荷转移过程的关键因素的广泛研究工作的主题。以前,我们已经观察到,具有 Tp = 三(吡唑基)硼酸盐和 vbik = 双(1-乙烯基-2-咪唑基)酮的方配合物 {[Fe(Tp)(CN)3]2[Co(vbik)2]2+,缩写为 {Fe2Co2},在接近室温的 MeOH 溶液中发生热电荷转移,从而获得溶剂晶型对,{FeIII2CoII2}·2BF4·2MeOH (1) 和 {FeII2CoIII2}·2BF4·10H2O·2MeOH (2),在 300 K 时表现出不同的电子构型。虽然 2 在固态下保持其电荷转移能力,但 1 在顺磁状态中被低至 2 K 的固相相互作用捕获,这使其成为研究静水压力下电子转移的良好候选者,静水压力是这些系统很少使用的外部刺激。在本工作中,我们证明了合成方法可用于获得具有显着压力诱导电子转移的新型溶剂晶型。 顺磁性 {FeIII2CoII2}·2(PF6)·2MeOH (3) 和抗磁性 {FeII2CoIII2}·2(PF6)·nH2O·米获得了 MeOH (4),分别与 1 和 2 同构。由于 PF6 阴离子引起的更强分子间相互作用导致 3 和 4 的核心结构发生更大的变形,从而影响它们在环境和静水压力下的磁性。值得注意的是,3 表现出从顺磁状态到抗磁状态的分压诱导转换,然后是高于压力阈值的反向转换,这可以通过大约 0.96–1.0 GPa 的对称性打破相变来合理化。在各种压力下,已经使用磁力计、X 射线衍射和 μ-Raman 光谱分析了这种不寻常的行为。通过分析其在各种压力下的结构数据并将其与 1 的结构数据进行比较,可以更深入地了解 3 中的电子转移过程。在两种配合物中观察到的不同电子转移行为可能与压力引起的方形核心结构的不同扭曲有关。






























 京公网安备 11010802027423号
京公网安备 11010802027423号