当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Scale-up of a Sulfonamide Catalyst for Mercaptan Removal from Light Oils: A Reaction Kinetics Investigation with Density Functional Theory Validation
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-27 , DOI: 10.1021/acs.iecr.4c03125 Sudip K. Ganguly, Sunil Kumar, Dev Choudhary, Vivek Rathore, Bharat L. Newalkar, Vivek K. Chaudhary, Yashika Sharma, Ashwinth K, Anjan Ray, Tuhin S. Khan
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-27 , DOI: 10.1021/acs.iecr.4c03125 Sudip K. Ganguly, Sunil Kumar, Dev Choudhary, Vivek Rathore, Bharat L. Newalkar, Vivek K. Chaudhary, Yashika Sharma, Ashwinth K, Anjan Ray, Tuhin S. Khan
|
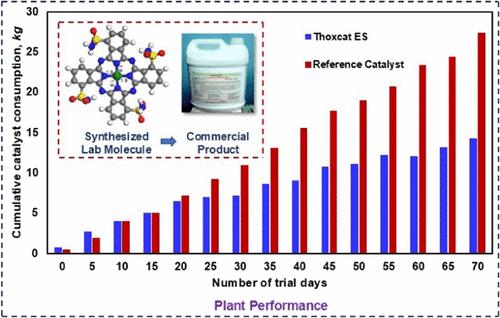
The sweetening of lighter petroleum fractions such as liquefied petroleum gas (LPG) involves the extraction of lighter thiols (RSHs) by caustication to form mercaptides (NaSRs). Oxygen subsequently oxidizes these NaSRs to disulfides (RSSRs) in the presence of a catalyst typically – based on cobalt phthalocyanines (CoPc) and an alkali. The present study focuses on joint development and commercialization efforts for an LPG sweetening catalyst – Thoxcat ES – by CSIR-Indian Institute of Petroleum (IIP) and Bharat Petroleum Corporation Ltd. (BPCL) using a step-by-step approach toward elucidating mechanistic pathways, establishing applicable rate laws, laboratory reactor design, and determining kinetic parameters for the catalyst–substrate system. Gas–liquid (G-L) kinetics studies using oxygen were conducted in an agitated and sparged tank contactor (ASTC) equipped with a turbine impeller operated in the total recirculation regime (TRR) using 1-ethanethiol in the range of 400–700 ppm w at temperature (T) = 303–317 K and atmospheric pressure. The derived rate law, though similar to the Michaelis–Menten rate law, simplifies into a linear form for the usual industrial process conditions. The experimental activation energy (Ea) determined as 50.715 kJ mol–1 compared within a range of ±2.0% with density functional theory (DFT) predictions indicates adequate representation of intrinsic kinetics after discounting hydrodynamic and mass transfer limitations. During refinery runs, such kinetic models are helpful in optimization of catalyst makeup dosages and dosing frequency for achieving operating cost competitiveness in petroleum refineries.
中文翻译:

用于从轻油中去除硫醇的磺酰胺催化剂的合成和放大:具有密度泛函理论验证的反应动力学研究
较轻的石油馏分(如液化石油气 (LPG))的脱硫涉及通过苛化提取较轻的硫醇 (RSH) 以形成硫醇 (NaSR)。随后,氧气在催化剂存在下将这些 NaSR 氧化成二硫化物 (RSSR) – 通常基于钴酞菁 (CoPc) 和碱。本研究的重点是 CSIR-印度石油研究所 (IIP) 和 Bharat 石油公司有限公司 (BPCL) 联合开发和商业化液化石油气脱硫催化剂 – Thoxcat ES,使用分步方法来阐明机理途径、建立适用的速率定律、实验室反应器设计以及确定催化剂-底板系统的动力学参数。使用氧气的气液 (G-L) 动力学研究在搅拌和喷射罐接触器 (ASTC) 中进行,该接触器配备涡轮叶轮,在温度 (T) = 303-317 K 和大气压下使用 1-乙硫醇在 400-700 ppm w 范围内在全再循环状态 (TRR) 中运行。推导的速率定律虽然类似于 Michaelis-Menten 速率定律,但对于通常的工业过程条件,它被简化为线性形式。在密度泛函理论 (DFT) 预测的 ±2.0% 范围内,确定为 50.715 kJ mol–1 的实验活化能 (Ea) 表明,在扣除流体动力学和传质限制后,本征动力学的充分表示。在炼油厂运行期间,这种动力学模型有助于优化催化剂补充剂量和投加频率,从而在炼油厂中实现运营成本竞争力。
更新日期:2024-11-27
中文翻译:

用于从轻油中去除硫醇的磺酰胺催化剂的合成和放大:具有密度泛函理论验证的反应动力学研究
较轻的石油馏分(如液化石油气 (LPG))的脱硫涉及通过苛化提取较轻的硫醇 (RSH) 以形成硫醇 (NaSR)。随后,氧气在催化剂存在下将这些 NaSR 氧化成二硫化物 (RSSR) – 通常基于钴酞菁 (CoPc) 和碱。本研究的重点是 CSIR-印度石油研究所 (IIP) 和 Bharat 石油公司有限公司 (BPCL) 联合开发和商业化液化石油气脱硫催化剂 – Thoxcat ES,使用分步方法来阐明机理途径、建立适用的速率定律、实验室反应器设计以及确定催化剂-底板系统的动力学参数。使用氧气的气液 (G-L) 动力学研究在搅拌和喷射罐接触器 (ASTC) 中进行,该接触器配备涡轮叶轮,在温度 (T) = 303-317 K 和大气压下使用 1-乙硫醇在 400-700 ppm w 范围内在全再循环状态 (TRR) 中运行。推导的速率定律虽然类似于 Michaelis-Menten 速率定律,但对于通常的工业过程条件,它被简化为线性形式。在密度泛函理论 (DFT) 预测的 ±2.0% 范围内,确定为 50.715 kJ mol–1 的实验活化能 (Ea) 表明,在扣除流体动力学和传质限制后,本征动力学的充分表示。在炼油厂运行期间,这种动力学模型有助于优化催化剂补充剂量和投加频率,从而在炼油厂中实现运营成本竞争力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号