当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regeneration of Spent Desiccants with Supercritical CO2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-27 , DOI: 10.1021/acs.iecr.4c03636 Astrid Melissa Rojas Márquez, Iris Beatriz Vega Erramuspe, Brian K. Via, Bhima Sastri, Sujit Banerjee
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-27 , DOI: 10.1021/acs.iecr.4c03636 Astrid Melissa Rojas Márquez, Iris Beatriz Vega Erramuspe, Brian K. Via, Bhima Sastri, Sujit Banerjee
|
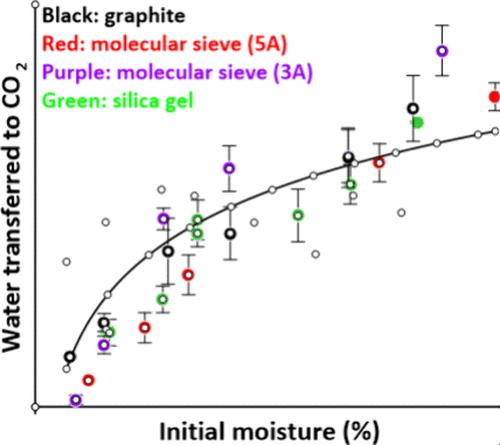
Supercritical CO2 (sCO2) dehydrates desiccants such as silica gel, activated carbon, graphite, and molecular sieve by dissolving and emulsifying the water. Despite differences in the surface area of these desiccants, the amount of water removed under comparable conditions is the same. The main advantage of sCO2 dewatering over conventional hot-air regeneration lies in situations where the exhaust contains environmentally sensitive components, e.g., in nuclear detritiation operations where the small footprint and closed cycle benefits of the sCO2 process are especially significant. Calculations show that depressurizing the spent sCO2 to half its initial pressure drops out most of the water, after which the CO2 can be repressurized and reused. sCO2 dewatering requires about half the energy needed for thermal drying because the water is removed nonevaporatively.
中文翻译:

用超临界 CO2 再生废干燥剂
超临界 CO2 (sCO2) 通过溶解和乳化水来脱水硅胶、活性炭、石墨和分子筛等干燥剂。尽管这些干燥剂的表面积不同,但在同等条件下去除的水量是相同的。与传统的热空气再生相比,sCO2 脱水的主要优势在于废气中含有环境敏感成分的情况,例如,在核氚化操作中,sCO2 工艺的小占地面积和闭式循环优势尤为显著。计算表明,将用过的 sCO2 减压至其初始压力的一半会使大部分水流失,之后 CO2 可以重新加压和再利用。sCO2 脱水需要的能量大约是热干燥所需能量的一半,因为水是非蒸发去除的。
更新日期:2024-11-27
中文翻译:

用超临界 CO2 再生废干燥剂
超临界 CO2 (sCO2) 通过溶解和乳化水来脱水硅胶、活性炭、石墨和分子筛等干燥剂。尽管这些干燥剂的表面积不同,但在同等条件下去除的水量是相同的。与传统的热空气再生相比,sCO2 脱水的主要优势在于废气中含有环境敏感成分的情况,例如,在核氚化操作中,sCO2 工艺的小占地面积和闭式循环优势尤为显著。计算表明,将用过的 sCO2 减压至其初始压力的一半会使大部分水流失,之后 CO2 可以重新加压和再利用。sCO2 脱水需要的能量大约是热干燥所需能量的一半,因为水是非蒸发去除的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号