当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular Chiral Bithiophene-2NO Ligands: Synthesis and Application in Asymmetric Palladium(II)-Catalysed Friedel-Crafts Alkylation
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-25 , DOI: 10.1002/adsc.202401348 Xian-Qiao Zhu, Wu-Wu Li, Lin-Lu Liu, Ke-Lan Xu, Li-Jun Peng, Min Zhang, Xiong-Li Liu, Wen-Jing Zhang
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-25 , DOI: 10.1002/adsc.202401348 Xian-Qiao Zhu, Wu-Wu Li, Lin-Lu Liu, Ke-Lan Xu, Li-Jun Peng, Min Zhang, Xiong-Li Liu, Wen-Jing Zhang
|
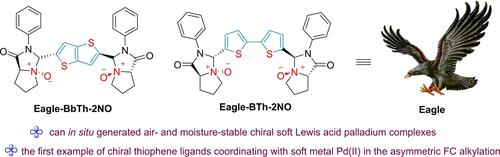
The development of privileged thiophene-type ligand remains highly desirable, owing to the high coordination ability of sulfur atom to most of the soft metals. Herein, a new class of C2-symmetric rigid chiral tertiary amine-derived dioxide ligands with a coordinating bithiophene bridge was developed in a convenient synthetic route with 25–62% overall yields. The bithiophene-2NO-palladium(II) catalyst system was generated in situ and found to be highly capable catalyst in the asymmetric Friedel-Crafts alkylation of indoles. Excellent yields (up to 92%) and high enantioselectivities (up to >99% ee) are obtained for a wide range of substrates under mild conditions. Control experiments and DFT calculations revealed the origins of the enantioselectivity. This represented the first example of chiral thiophene ligands coordinating with soft metal Pd(II) in the asymmetric Friedel-Crafts alkylation. Experiments revealed that the counteranion is involved in the stereoselectivity-determining step in this soft palladium(II) catalysis, and the additional NO and thiophene in ligands can act as the second chelation site and thus reduce the amount of free metal ions for suppressing background reaction in the catalytic system.
中文翻译:

模块化手性联噻吩-2NO 配体:在不对称钯 (II) 催化的 Friedel-Crafts 烷基化反应中的合成和应用
由于硫原子对大多数软金属的高配位能力,特权噻吩型配体的发展仍然非常可取。在此,以方便的合成路线开发了一类新的 C2 对称刚性手性叔胺衍生的二氧化二元配体,具有配位联噻吩桥,总产率为 25-62%。原位生成二噻吩-2NO-钯 (II) 催化剂系统,发现在吲哚的不对称 Friedel-Crafts 烷基化反应中是高性能催化剂。在温和条件下,对各种底物均获得出色的产量(高达 92%)和高对映体选择性(高达 >99% ee)。对照实验和 DFT 计算揭示了对映选择性的起源。这代表了手性噻吩配体在不对称 Friedel-Crafts 烷基化中与软金属 Pd(II) 配位的第一个例子。实验表明,反阴离子参与这种软钯 (II) 催化中的立体选择性决定步骤,配体中额外的 NO 和噻吩可以作为第二个螯合位点,从而减少催化系统中用于抑制背景反应的自由金属离子的量。
更新日期:2024-11-25
中文翻译:

模块化手性联噻吩-2NO 配体:在不对称钯 (II) 催化的 Friedel-Crafts 烷基化反应中的合成和应用
由于硫原子对大多数软金属的高配位能力,特权噻吩型配体的发展仍然非常可取。在此,以方便的合成路线开发了一类新的 C2 对称刚性手性叔胺衍生的二氧化二元配体,具有配位联噻吩桥,总产率为 25-62%。原位生成二噻吩-2NO-钯 (II) 催化剂系统,发现在吲哚的不对称 Friedel-Crafts 烷基化反应中是高性能催化剂。在温和条件下,对各种底物均获得出色的产量(高达 92%)和高对映体选择性(高达 >99% ee)。对照实验和 DFT 计算揭示了对映选择性的起源。这代表了手性噻吩配体在不对称 Friedel-Crafts 烷基化中与软金属 Pd(II) 配位的第一个例子。实验表明,反阴离子参与这种软钯 (II) 催化中的立体选择性决定步骤,配体中额外的 NO 和噻吩可以作为第二个螯合位点,从而减少催化系统中用于抑制背景反应的自由金属离子的量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号