当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atom-Economical and Environmentally Friendly Bts-Based Purine PNA Monomers without Base-Protecting Groups
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-25 , DOI: 10.1021/acs.oprd.4c00413 Minji Kim, Hyewon Hwang, Yong-Tae Kim, In Seok Hong
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-25 , DOI: 10.1021/acs.oprd.4c00413 Minji Kim, Hyewon Hwang, Yong-Tae Kim, In Seok Hong
|
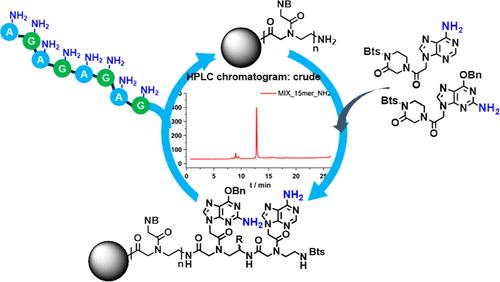
Traditionally, Fmoc/Bhoc or Bts/Bhoc monomers have been employed in the solid-phase peptide synthesis (SPPS) of peptide nucleic acids (PNAs). The primary function of the Bhoc group as a base-protecting group is to prevent the exocyclic amine of the nucleobase from participating in the coupling reaction and to enhance the solubility of the monomer in the coupling solvent. However, regarding the nucleophilicity of the base, only the exocyclic amine at C4 of cytosine exhibits significant nucleophilicity, while the exocyclic amines of adenine (N6) and guanine (N2) have minimal nucleophilic character. In fact, the protection of the exocyclic amine in purine bases during SPPS is unnecessary if the sole objective is to improve the monomer’s solubility. In this study, we synthesized novel purine monomers based on cyclic Bts that lack base-protecting groups. Using these unprotected monomers, PNA oligomers were synthesized via the SPPS method and the crude purities of these oligomers were compared with those synthesized using fully Bhoc-protected monomers. The crude purity of 15-mer PNA oligomers using six purine monomers without protecting groups was superior to that of oligomers synthesized from Bts/Bhoc monomers. Moreover, we demonstrated that these unprotected monomers are effective for synthesizing poly purine PNA oligomers, which are otherwise challenging to synthesize by SPPS. As a result, we have synthesized the first Bts-based purine monomers without base-protecting groups, offering a more atom-efficient and environmentally friendly alternative to conventional Bhoc-protected purine monomers for large-scale PNA oligomer production.
中文翻译:

原子经济且环保的基于 Bts 的嘌呤 PNA 单体,不含碱基保护基团
传统上,Fmoc/Bhoc 或 Bts/Bhoc 单体已用于肽核酸 (PNA) 的固相肽合成 (SPPS)。Bhoc 基团作为碱基保护基团的主要功能是防止核碱基的外环胺参与偶联反应,并提高单体在偶联溶剂中的溶解度。然而,关于碱基的亲核性,只有胞嘧啶 C4 的外环胺表现出显着的亲核性,而腺嘌呤 (N6) 和鸟嘌呤 (N2) 的外环胺具有最小的亲核特性。事实上,如果唯一目的是提高单体的溶解度,那么在 SPPS 过程中保护嘌呤碱中的外环胺是不必要的。在这项研究中,我们合成了基于缺乏碱基保护基团的环状 Bts 的新型嘌呤单体。使用这些未受保护的单体,通过 SPPS 方法合成 PNA 低聚物,并将这些低聚物的粗纯度与使用完全 Bhoc 保护的单体合成的低聚物进行比较。使用不含保护基团的 6 个嘌呤单体的 15 聚体 PNA 低聚物的粗纯度优于由 Bts/Bhoc 单体合成的低聚物。此外,我们证明这些未受保护的单体可有效合成聚嘌呤 PNA 寡聚体,否则 SPPS 合成这些寡聚物将具有挑战性。因此,我们合成了第一个不含碱基保护基团的基于 Bts 的嘌呤单体,为大规模 PNA 低聚物生产提供了比传统 Bhoc 保护嘌呤单体更具原子效率和环保性的替代方案。
更新日期:2024-11-25
中文翻译:

原子经济且环保的基于 Bts 的嘌呤 PNA 单体,不含碱基保护基团
传统上,Fmoc/Bhoc 或 Bts/Bhoc 单体已用于肽核酸 (PNA) 的固相肽合成 (SPPS)。Bhoc 基团作为碱基保护基团的主要功能是防止核碱基的外环胺参与偶联反应,并提高单体在偶联溶剂中的溶解度。然而,关于碱基的亲核性,只有胞嘧啶 C4 的外环胺表现出显着的亲核性,而腺嘌呤 (N6) 和鸟嘌呤 (N2) 的外环胺具有最小的亲核特性。事实上,如果唯一目的是提高单体的溶解度,那么在 SPPS 过程中保护嘌呤碱中的外环胺是不必要的。在这项研究中,我们合成了基于缺乏碱基保护基团的环状 Bts 的新型嘌呤单体。使用这些未受保护的单体,通过 SPPS 方法合成 PNA 低聚物,并将这些低聚物的粗纯度与使用完全 Bhoc 保护的单体合成的低聚物进行比较。使用不含保护基团的 6 个嘌呤单体的 15 聚体 PNA 低聚物的粗纯度优于由 Bts/Bhoc 单体合成的低聚物。此外,我们证明这些未受保护的单体可有效合成聚嘌呤 PNA 寡聚体,否则 SPPS 合成这些寡聚物将具有挑战性。因此,我们合成了第一个不含碱基保护基团的基于 Bts 的嘌呤单体,为大规模 PNA 低聚物生产提供了比传统 Bhoc 保护嘌呤单体更具原子效率和环保性的替代方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号