当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular assembly of chiral multisubstituted tetrahydropyrans through a cascade asymmetric aldehyde allylboration/oxa-cyclization by a chiral phosphoric acid
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-26 , DOI: 10.1039/d4qo01851f Yu-Hao Wang, Bo-Jun He, Subarna Jyoti Kalita, Zhen-Ni Zhao, Yi-Chao Li, De-Hua Zhang, Zi-Qi Yi, Uwe Schneider, Yi-Yong Huang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-26 , DOI: 10.1039/d4qo01851f Yu-Hao Wang, Bo-Jun He, Subarna Jyoti Kalita, Zhen-Ni Zhao, Yi-Chao Li, De-Hua Zhang, Zi-Qi Yi, Uwe Schneider, Yi-Yong Huang
|
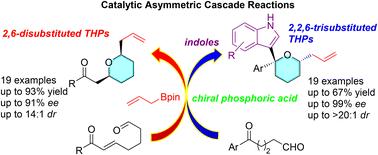
Considering the structural importance of 2,6-disubstituted and 2,2,6-trisubstituted tetrahydropyran motifs, two novel types of cascade reactions have been developed to address the challenges of synthetic efficiency, high stereoselectivity and construction of a diaryl quaternary stereogenic carbon center. A single BINOL-derived chiral phosphoric acid catalyst enabled the asymmetric aldehyde-allylboration and subsequent intramolecular oxa-Michael addition or intermolecular variant of oxa-Pictet–Spengler reactions with high to excellent yields (up to 93%), diastereoselectivities (up to >20 : 1 dr) and enantioselectivities (up to 99% ee). The origin of the stereochemical induction was understood via chair-like 6-membered transition states and DFT calculations, which suggested that the thermodynamically favored 2,2,6-trisubstituted THPs were obtained through the kinetically controlled attack of indole on the Si-face of the oxocarbenium ion intermediate.
中文翻译:

手性多取代四氢吡喃通过手性磷酸的级联不对称醛烯丙基化/氧代环化实现手性多取代四氢吡喃的模块化组装
考虑到 2,6-二取代和 2,2,6-三取代四氢吡喃基序的结构重要性,已经开发了两种新型的级联反应,以应对合成效率、高立体选择性和二芳基季叔立体碳中心构建的挑战。单个 BINO 衍生的手性磷酸催化剂实现了不对称的醛-烯丙基硼化和随后的分子内 oxa-Michael 加成或 oxa-Pictet-Spengler 反应的分子间变体,具有高产率(高达 93%)、非对映选择性(高达 >20 : 1 dr)和对映选择性(高达 99% ee)。立体化学诱导的起源是通过椅子状 6 元过渡态和 DFT 计算来理解的,这表明热力学上有利的 2,2,6-三取代 THP 是通过吲哚对氧碳烯离子中间体 Si 面的动力学控制攻击获得的。
更新日期:2024-11-26
中文翻译:

手性多取代四氢吡喃通过手性磷酸的级联不对称醛烯丙基化/氧代环化实现手性多取代四氢吡喃的模块化组装
考虑到 2,6-二取代和 2,2,6-三取代四氢吡喃基序的结构重要性,已经开发了两种新型的级联反应,以应对合成效率、高立体选择性和二芳基季叔立体碳中心构建的挑战。单个 BINO 衍生的手性磷酸催化剂实现了不对称的醛-烯丙基硼化和随后的分子内 oxa-Michael 加成或 oxa-Pictet-Spengler 反应的分子间变体,具有高产率(高达 93%)、非对映选择性(高达 >20 : 1 dr)和对映选择性(高达 99% ee)。立体化学诱导的起源是通过椅子状 6 元过渡态和 DFT 计算来理解的,这表明热力学上有利的 2,2,6-三取代 THP 是通过吲哚对氧碳烯离子中间体 Si 面的动力学控制攻击获得的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号