当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using CrN4 moiety to weaken the dissociation barrier of hydroxyl on adjacent single iron atom for efficient oxygen reduction
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-23 , DOI: 10.1016/j.ensm.2024.103927 Lilian Wang, Li Yang, Xinyu Zhao, Hang Ma, Bohuai Pang, Lingyan Duan, Kun Zeng, Lu Liu, Anran Chen, Hong Guo
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-23 , DOI: 10.1016/j.ensm.2024.103927 Lilian Wang, Li Yang, Xinyu Zhao, Hang Ma, Bohuai Pang, Lingyan Duan, Kun Zeng, Lu Liu, Anran Chen, Hong Guo
|
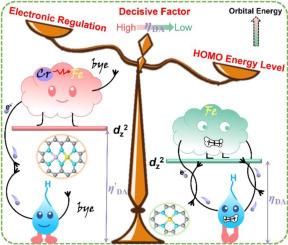
The strong perturbation of the valence band within the entire material system and accelerated *OH dissociation from Fe site are triggered by incorporating CrN4 moiety with low Fenton effect and *OH adsorption energy. This atomically dispersed Cr=N2 =Fe electrocatalyst is developed by adopting a super time-/energy-saving Joule heating strategy (∼ 10 s). Through the investigation of valence orbital energy levels and valence electron behavior for the prepared catalysts, in combination with in-situ Raman testing and theoretical calculations, we have determined that the interaction between the metal sites and oxygen-containing intermediates primarily depends on orbital energy levels before being further evaluated by bond order involving electronic modulation. This finding may offer a valuable insight for future research in related electrocatalysis fields. The Cr=N2 =Fe catalyst exhibits higher ORR catalytic capability than commercial Pt/C, thus driving stable operation of the assembled zinc-air battery for over 300 h.
中文翻译:

使用 CrN4 部分削弱羟基在相邻单个铁原子上的解离屏障,以实现有效的氧还原
通过掺入具有低 Fenton 效应和 *OH 吸附能的 CrN4 部分,触发了整个材料体系内价带的强烈扰动和加速 *OH 与 Fe 位点的解离。这种原子分散的 Cr=N2=Fe 电催化剂是通过采用超省时/节能的焦耳热策略 (∼ 10 s) 开发的。通过对所制备催化剂的价轨道能级和价电子行为的研究,结合原位拉曼测试和理论计算,我们确定金属位点和含氧中间体之间的相互作用主要取决于轨道能级,然后通过涉及电子调制的键序进一步评估。这一发现可能为相关电催化领域的未来研究提供有价值的见解。Cr=N2=Fe 催化剂表现出比商用 Pt/C 更高的 ORR 催化能力,从而驱动组装好的锌空气电池稳定运行超过 300 小时。
更新日期:2024-11-23
中文翻译:

使用 CrN4 部分削弱羟基在相邻单个铁原子上的解离屏障,以实现有效的氧还原
通过掺入具有低 Fenton 效应和 *OH 吸附能的 CrN4 部分,触发了整个材料体系内价带的强烈扰动和加速 *OH 与 Fe 位点的解离。这种原子分散的 Cr=N2=Fe 电催化剂是通过采用超省时/节能的焦耳热策略 (∼ 10 s) 开发的。通过对所制备催化剂的价轨道能级和价电子行为的研究,结合原位拉曼测试和理论计算,我们确定金属位点和含氧中间体之间的相互作用主要取决于轨道能级,然后通过涉及电子调制的键序进一步评估。这一发现可能为相关电催化领域的未来研究提供有价值的见解。Cr=N2=Fe 催化剂表现出比商用 Pt/C 更高的 ORR 催化能力,从而驱动组装好的锌空气电池稳定运行超过 300 小时。






























 京公网安备 11010802027423号
京公网安备 11010802027423号