当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trade-off effect of Fe in earth-abundant P2-type sodium cathode materials: Transition metal dissolution and oxygen redox mechanism
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.ensm.2024.103924 Hye-Jin Kim, Natalia Voronina, Jun Ho Yu, Vitalii A. Shevchenko, Oleg A. Drozhzhin, Ko-Eun Ryou, Eui-Yeon Jeong, A-Yeon Kim, Hyeon-Ji Shin, Hun-Gi Jung, Kyuwook lhm, Kug-Seung Lee, Koji Yazawa, Hitoshi Yashiro, Seong-Min Bak, Igor A. Presniakov, Evgeny V. Antipov, Seung-Taek Myung
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.ensm.2024.103924 Hye-Jin Kim, Natalia Voronina, Jun Ho Yu, Vitalii A. Shevchenko, Oleg A. Drozhzhin, Ko-Eun Ryou, Eui-Yeon Jeong, A-Yeon Kim, Hyeon-Ji Shin, Hun-Gi Jung, Kyuwook lhm, Kug-Seung Lee, Koji Yazawa, Hitoshi Yashiro, Seong-Min Bak, Igor A. Presniakov, Evgeny V. Antipov, Seung-Taek Myung
|
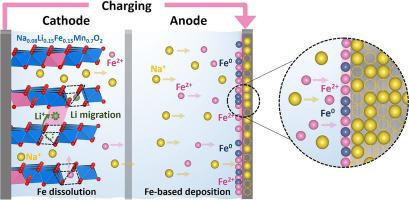
P2-Nax [Liy Mn1−y−z ]O2 (TM: Ni, Cu, Co, Fe) exhibits anionic redox reactions, and among these, the cost-effective Fe substitution has attracted significant attention to aid reasonable a promising material. However, we find that the dissolution and deposition of Fe present in the TM layer can cause detrimental effects, structural disintegration that affects capacity fading. Operando X-ray diffraction shows that the P2 phase of Na0.6 [Li0.15 Fe0.15 Mn0.7 ]O2 is maintained during de/sodiation processes, and X-ray absorption analysis reveals the redox activity of Fe3+ /Fe4+ , Mn3+ /Mn4+ , and O2− /(O2 )n− redox pairs. Mössbauer spectroscopy provides insights into the behavior of Fe during de/sodiation, particularly in the two-electron oxidation process observed during charging, where the Fe³⁺/Fe⁴⁺ redox reaction simultaneously influences the oxidation of lattice oxygen, thereby aiding overall charge compensation during desodiation. These findings clarify the connection between this process and the redox activity of lattice oxygen. Additionally, 7 Li NMR is employed to analyze the migration of Li from the transition-metal layer to the Na layer, elucidating the anionic-redox-reaction mechanism. Notably, X-ray photoelectron spectroscopy and inductively coupled plasma–atomic emission spectroscopy analyses demonstrate Fe dissolution and subsequent deposition on the surface of anode, leading to capacity degradation and poor electrochemical performance. These findings underscore the significant impact of Fe dissolution and deposition on the performance of Na0.6 [Li0.15 Fe0.15 Mn0.7 ]O2 , highlighting the challenges associated with Fe doping in cathode materials for sodium-ion batteries.
中文翻译:

Fe 在地球上丰富的 P2 型钠正极材料中的权衡效应:过渡金属溶解和氧氧化还原机理
P2-Nax[LiyMn1−y−z]O2 (TM: Ni, Cu, Co, Fe) 表现出阴离子氧化还原反应,其中,具有成本效益的 Fe 取代引起了极大的关注,以帮助合理地成为一种有前途的材料。然而,我们发现 TM 层中存在的 Fe 的溶解和沉积会导致有害影响,即影响容量衰减的结构分解。原位 X 射线衍射显示,Na0.6[Li0.15Fe0.15Mn0.7]O2 的 P2 相在脱/钠化过程中保持不变,X 射线吸收分析揭示了 Fe3+/Fe4+、Mn3+/Mn4+ 和 O2−/(O2)n−氧化还原对的氧化还原活性。穆斯堡尔光谱可以深入了解 Fe 在脱钠过程中的行为,特别是在充电过程中观察到的双电子氧化过程中,其中 Fe³⁺/Fe⁴⁺ 氧化还原反应同时影响晶格氧的氧化,从而有助于脱盐过程中的整体电荷补偿。这些发现阐明了这个过程与晶格氧的氧化还原活性之间的联系。此外,7Li NMR 用于分析锂从过渡金属层到 Na 层的迁移,阐明阴离子氧化还原反应机制。值得注意的是,X 射线光电子能谱和电感耦合等离子体-原子发射光谱分析表明 Fe 溶解并随后沉积在阳极表面,导致容量退化和电化学性能差。这些发现强调了 Fe 溶解和沉积对 Na0.6[Li0.15Fe0.15Mn0.7]O2 性能的显着影响,突出了钠离子电池正极材料中 Fe 掺杂的相关挑战。
更新日期:2024-11-22
中文翻译:

Fe 在地球上丰富的 P2 型钠正极材料中的权衡效应:过渡金属溶解和氧氧化还原机理
P2-Nax[LiyMn1−y−z]O2 (TM: Ni, Cu, Co, Fe) 表现出阴离子氧化还原反应,其中,具有成本效益的 Fe 取代引起了极大的关注,以帮助合理地成为一种有前途的材料。然而,我们发现 TM 层中存在的 Fe 的溶解和沉积会导致有害影响,即影响容量衰减的结构分解。原位 X 射线衍射显示,Na0.6[Li0.15Fe0.15Mn0.7]O2 的 P2 相在脱/钠化过程中保持不变,X 射线吸收分析揭示了 Fe3+/Fe4+、Mn3+/Mn4+ 和 O2−/(O2)n−氧化还原对的氧化还原活性。穆斯堡尔光谱可以深入了解 Fe 在脱钠过程中的行为,特别是在充电过程中观察到的双电子氧化过程中,其中 Fe³⁺/Fe⁴⁺ 氧化还原反应同时影响晶格氧的氧化,从而有助于脱盐过程中的整体电荷补偿。这些发现阐明了这个过程与晶格氧的氧化还原活性之间的联系。此外,7Li NMR 用于分析锂从过渡金属层到 Na 层的迁移,阐明阴离子氧化还原反应机制。值得注意的是,X 射线光电子能谱和电感耦合等离子体-原子发射光谱分析表明 Fe 溶解并随后沉积在阳极表面,导致容量退化和电化学性能差。这些发现强调了 Fe 溶解和沉积对 Na0.6[Li0.15Fe0.15Mn0.7]O2 性能的显着影响,突出了钠离子电池正极材料中 Fe 掺杂的相关挑战。






























 京公网安备 11010802027423号
京公网安备 11010802027423号