当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of counterions on the thermal and solution properties of strong polyelectrolytes
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-23 , DOI: 10.1039/d4py01218f Théophile Pelras, Julien Es Sayed, Jin Pierik, Andrea Giuntoli, Anton H. Hofman, Katja Loos, Marleen Kamperman
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-23 , DOI: 10.1039/d4py01218f Théophile Pelras, Julien Es Sayed, Jin Pierik, Andrea Giuntoli, Anton H. Hofman, Katja Loos, Marleen Kamperman
|
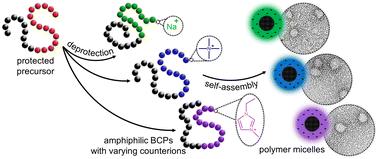
Strong polyelectrolytes (i.e., macromolecules whose charge density is independent of the medium's pH) are invaluable assets in the soft matter toolbox, as they can readily disperse in aqueous media, complex to oppositely charged species – polymers and small molecules alike – and can be implemented in a plethora of applications, ranging from surface modification to chelating agents and lubricants. However, the direct synthesis of strong polyelectrolytes in a controlled fashion remains a challenging endeavour, and their in-depth characterisation is often limited. Additionally, producing a set of charged macromolecules with the same chain length but varying counterions would open doors towards a fine control of the polymer's chemistry and physical properties. Unfortunately, this either necessitates the direct polymerisation of several monomers with potentially varying reactivities, or a time-consuming ion exchange from a single batch. Herein we explore the facile and efficient production of strong polyanions through the deprotection of a poly(3-isobutoxysulphopropyl methacrylate) using a range of inorganic and organic iodide-containing salts. Owing to the contrasting nature of their counterions, the resulting polyanions exhibit a wide range of glass transition temperatures, which follow a non-monotonic trend with increasing counterion size. While all polymers readily dissolve in water, some can also be dissolved in non-aqueous media as well. This strategy, applied to block copolymers, permits the production of a library of amphiphilic macromolecules with consistent hydrophilic and hydrophobic blocks, yet varying nature of their polyanionic segments. All amphiphiles, regardless of their counterions, readily disperse in aqueous media and form well-defined micelles featuring a hydrophobic core and a charged hydrophilic shell, as evidenced by dynamic light scattering, ζ-potential and transmission electron microscopy. Additionally, a handful of block copolymers are capable of yielding polymer micelles in organic solvents, opening an avenue to the build-up of nanostructured soft matter in non-aqueous media.
中文翻译:

反离子对强聚电解质的热性能和溶液性能的影响
强聚电解质(即电荷密度与介质 pH 值无关的大分子)是软物质工具箱中的宝贵资产,因为它们可以很容易地分散在水性介质中,从复杂到带相反电荷的物质(聚合物和小分子)中,并且可以在从表面改性到螯合剂和润滑剂的众多应用中实现。然而,以受控方式直接合成强聚电解质仍然是一项具有挑战性的工作,并且它们的深入表征通常受到限制。此外,生产一组具有相同链长但不同反离子的带电大分子将为精细控制聚合物的化学和物理性质打开大门。不幸的是,这要么需要直接聚合几个可能具有不同反应性的单体,要么需要从单个批次进行耗时的离子交换。在本文中,我们探索了通过使用一系列含无机和有机碘化物的盐对聚(3-异丁氧基磺丙基甲基丙烯酸酯)进行脱保护来简单高效地生产强聚阴离子。由于它们的对离子的对比性质,所得的聚阴离子表现出很宽的玻璃化转变温度范围,随着对离子尺寸的增加,该温度呈非单调趋势。虽然所有聚合物都很容易溶于水,但有些聚合物也可以溶解在非水性介质中。该策略应用于嵌段共聚物,允许生产具有一致的亲水性和疏水性嵌段的两亲性大分子库,但其聚阴离子链段的性质不同。 所有两亲物,无论其反离子如何,都很容易分散在水性介质中,并形成具有疏水核心和带电亲水壳的明确胶束,动态光散射、ζ电位和透射电子显微镜检查证明了这一点。此外,少数嵌段共聚物能够在有机溶剂中产生聚合物胶束,为纳米结构软物质在非水性介质中的积累开辟了一条途径。
更新日期:2024-11-23
中文翻译:

反离子对强聚电解质的热性能和溶液性能的影响
强聚电解质(即电荷密度与介质 pH 值无关的大分子)是软物质工具箱中的宝贵资产,因为它们可以很容易地分散在水性介质中,从复杂到带相反电荷的物质(聚合物和小分子)中,并且可以在从表面改性到螯合剂和润滑剂的众多应用中实现。然而,以受控方式直接合成强聚电解质仍然是一项具有挑战性的工作,并且它们的深入表征通常受到限制。此外,生产一组具有相同链长但不同反离子的带电大分子将为精细控制聚合物的化学和物理性质打开大门。不幸的是,这要么需要直接聚合几个可能具有不同反应性的单体,要么需要从单个批次进行耗时的离子交换。在本文中,我们探索了通过使用一系列含无机和有机碘化物的盐对聚(3-异丁氧基磺丙基甲基丙烯酸酯)进行脱保护来简单高效地生产强聚阴离子。由于它们的对离子的对比性质,所得的聚阴离子表现出很宽的玻璃化转变温度范围,随着对离子尺寸的增加,该温度呈非单调趋势。虽然所有聚合物都很容易溶于水,但有些聚合物也可以溶解在非水性介质中。该策略应用于嵌段共聚物,允许生产具有一致的亲水性和疏水性嵌段的两亲性大分子库,但其聚阴离子链段的性质不同。 所有两亲物,无论其反离子如何,都很容易分散在水性介质中,并形成具有疏水核心和带电亲水壳的明确胶束,动态光散射、ζ电位和透射电子显微镜检查证明了这一点。此外,少数嵌段共聚物能够在有机溶剂中产生聚合物胶束,为纳米结构软物质在非水性介质中的积累开辟了一条途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号