当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Subconjunctival injection of microcrystalline prodrug of dexamethasone for long-acting anti-inflammation after phacoemulsification surgery
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-27 , DOI: 10.1016/j.jconrel.2024.11.046 Xueyan Zhou, Zunkai Xu, Yanliang Dong, Maoyu Cai, Zhixia Chen, Jingqing Mu, Bo Yuan, Xia Hua, Xiaoyong Yuan, Shutao Guo
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-27 , DOI: 10.1016/j.jconrel.2024.11.046 Xueyan Zhou, Zunkai Xu, Yanliang Dong, Maoyu Cai, Zhixia Chen, Jingqing Mu, Bo Yuan, Xia Hua, Xiaoyong Yuan, Shutao Guo
|
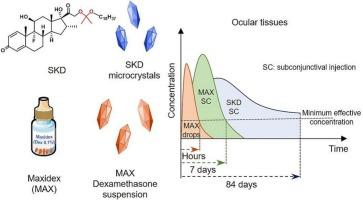
Long-acting injectable formulations of dexamethasone with minimal invasiveness are highly desired to manage chronic ocular inflammatory conditions. Here, we applied microcrystals (MCs) of a hydrophobic acetone-based ketal-linked prodrug of dexamethasone (SKD) to treat postoperative ocular inflammation. We compared the efficacy and safety of SKD MCs through subconjunctival (SC) injection with that of Maxidex (a topical suspension of dexamethasone MCs) through SC injection and eye drops in the phacoemulsification combined with intraocular lens implantation (Phaco-IOL) rabbit model. In Phaco-IOL rabbit eyes, a single SC injection of SKD MCs (0.4 mg dexamethasone equiv.) showed anti-inflammatory effects comparable to Maxidex eye drops and completely alleviated the inflammation within 28 days, while an SC injection of Maxidex at the same dose only provided anti-inflammatory effects for 7 days. The study on the dose-dependent anti-inflammatory effects of SKD MCs showed no significant difference in anti-inflammatory effects for the high dosage (0.8 mg dexamethasone equiv.) and the low dosage (0.4 mg dexamethasone equiv.) in 28 days. Nevertheless, systematic drug distribution of SKD MCs and Maxidex in normal rabbits after SC injection demonstrates that the drug concentration in conjunctiva was higher for the high dosage and that a considerable amount of prodrug and dexamethasone could still be detected in the cornea and iris-ciliary body at least 84 days for SKD MCs at high dosage. Furthermore, no persistent elevated intraocular pressure and abnormality in retinal structure and thickness were observed, confirming the excellent safety of long-acting SKD MCs post-SC injection. Our findings provide valuable insights into using prodrug-based MCs for treating ocular postoperative inflammation, and the detailed drug distribution analysis would promote the clinical translation of these MCs in ocular diseases.
中文翻译:

地塞米松微晶前药超声乳化手术后结膜下注射长效抗炎
极具侵入性的地塞米松长效注射制剂是治疗慢性眼炎性疾病的理想选择。在这里,我们应用了基于疏水性丙酮的酮酮连接前药地塞米松 (SKD) 的微晶 (MC) 来治疗术后眼部炎症。我们比较了通过结膜下 (SC) 注射 SKD MCs 与通过 SC 注射和滴眼液的 Maxidex (地塞米松 MCs 的局部混悬液) 在超声乳化联合人工晶状体植入术 (Phaco-IOL) 兔模型中的有效性和安全性。在 Phaco-IOL 兔眼中,单次皮下注射 SKD MCs(0.4 mg 地塞米松当量)显示出与 Maxidex 滴眼液相当的抗炎作用,并在 28 天内完全缓解炎症,而相同剂量的 Maxidex 皮下注射仅提供 7 天的抗炎作用。关于 SKD MCs 剂量依赖性抗炎作用的研究表明,高剂量 (0.8 mg 地塞米松当量) 和低剂量 (0.4 mg 地塞米松当量) 在 28 天内的抗炎作用没有显着差异。然而,SC 注射后 SKD MCs 和 Maxidex 在正常兔体内的系统药物分布表明,高剂量时结膜中的药物浓度较高,并且对于高剂量的 SKD MCs 至少 84 天,在角膜和虹膜睫状体中仍可检测到相当数量的前药和地塞米松。此外,未观察到持续升高的眼压以及视网膜结构和厚度的异常,证实了 SC 注射后长效 SKD MCs 的优异安全性。 我们的研究结果为使用基于前药的 MCs 治疗眼部术后炎症提供了有价值的见解,详细的药物分布分析将促进这些 MCs 在眼部疾病中的临床转化。
更新日期:2024-11-27
中文翻译:

地塞米松微晶前药超声乳化手术后结膜下注射长效抗炎
极具侵入性的地塞米松长效注射制剂是治疗慢性眼炎性疾病的理想选择。在这里,我们应用了基于疏水性丙酮的酮酮连接前药地塞米松 (SKD) 的微晶 (MC) 来治疗术后眼部炎症。我们比较了通过结膜下 (SC) 注射 SKD MCs 与通过 SC 注射和滴眼液的 Maxidex (地塞米松 MCs 的局部混悬液) 在超声乳化联合人工晶状体植入术 (Phaco-IOL) 兔模型中的有效性和安全性。在 Phaco-IOL 兔眼中,单次皮下注射 SKD MCs(0.4 mg 地塞米松当量)显示出与 Maxidex 滴眼液相当的抗炎作用,并在 28 天内完全缓解炎症,而相同剂量的 Maxidex 皮下注射仅提供 7 天的抗炎作用。关于 SKD MCs 剂量依赖性抗炎作用的研究表明,高剂量 (0.8 mg 地塞米松当量) 和低剂量 (0.4 mg 地塞米松当量) 在 28 天内的抗炎作用没有显着差异。然而,SC 注射后 SKD MCs 和 Maxidex 在正常兔体内的系统药物分布表明,高剂量时结膜中的药物浓度较高,并且对于高剂量的 SKD MCs 至少 84 天,在角膜和虹膜睫状体中仍可检测到相当数量的前药和地塞米松。此外,未观察到持续升高的眼压以及视网膜结构和厚度的异常,证实了 SC 注射后长效 SKD MCs 的优异安全性。 我们的研究结果为使用基于前药的 MCs 治疗眼部术后炎症提供了有价值的见解,详细的药物分布分析将促进这些 MCs 在眼部疾病中的临床转化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号