当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric tandem reaction for the enantioselective synthesis of chiral oxindoles to construct CyK dyes
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.checat.2024.101188 Le Wang, Zi-Hao Li, Di Wu, Rui-Tian Ge, Jia Zhou, Yin-Feng Zhang, Shu-Yu Zhang
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.checat.2024.101188 Le Wang, Zi-Hao Li, Di Wu, Rui-Tian Ge, Jia Zhou, Yin-Feng Zhang, Shu-Yu Zhang
|
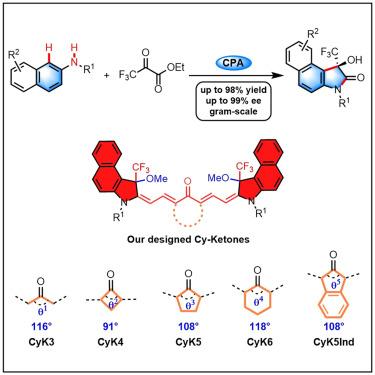
We report an efficient method for the synthesis of chiral 3-trifluoromethyl-3-hydroxy oxindoles through the asymmetric [3 + 2] cascade cyclization of simple 2-naphthylamine derivatives with ethyl trifluoropyruvate. This catalytic asymmetric strategy enables the efficient construction of a series of enantioenriched CF3-quaternary carbon oxindoles with high yields and excellent stereoselectivities. The innovative synthetic approach has been applied to the synthesis of trifluoromethylated Cy-ketone fluorescent dyes with circularly polarized luminescence (CPL) properties. In situ infrared and density functional theory calculations indicate that our catalytic system can overcome background reactions to achieve effective enantioselective annulation.
中文翻译:

用于手性氧吲哚的对映选择性合成以构建 CyK 染料的有机催化不对称串联反应
我们报道了一种通过简单的 2-萘胺衍生物与三氟丙酮酸乙酯的不对称 [3 + 2] 级联环化合成手性 3-三氟甲基-3-羟基氧吲哚的有效方法。这种催化不对称策略能够高效构建一系列对映体富集的 CF3-季铵碳吲哚,具有高产率和出色的立体选择性。创新的合成方法已应用于合成具有圆偏振发光 (CPL) 特性的三氟甲基化 Cy-酮荧光染料。原位红外和密度泛函理论计算表明,我们的催化系统可以克服背景反应,实现有效的对映选择性环化。
更新日期:2024-11-22
中文翻译:

用于手性氧吲哚的对映选择性合成以构建 CyK 染料的有机催化不对称串联反应
我们报道了一种通过简单的 2-萘胺衍生物与三氟丙酮酸乙酯的不对称 [3 + 2] 级联环化合成手性 3-三氟甲基-3-羟基氧吲哚的有效方法。这种催化不对称策略能够高效构建一系列对映体富集的 CF3-季铵碳吲哚,具有高产率和出色的立体选择性。创新的合成方法已应用于合成具有圆偏振发光 (CPL) 特性的三氟甲基化 Cy-酮荧光染料。原位红外和密度泛函理论计算表明,我们的催化系统可以克服背景反应,实现有效的对映选择性环化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号