Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Excited-state protonation and reduction enables the umpolung Birch reduction of naphthalenes
Chem ( IF 19.1 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.chempr.2024.10.009 Javier Corpas, Eva Rivera-Chao, Enrique M. Arpa, Miguel Gomez-Mendoza, Yuri Katayama, Victor A. de la Peña O’Shea, Céline Bouchel, Clément Jacob, Pierre-Georges Echeverria, Alessandro Ruffoni, Daniele Leonori
Chem ( IF 19.1 ) Pub Date : 2024-11-22 , DOI: 10.1016/j.chempr.2024.10.009 Javier Corpas, Eva Rivera-Chao, Enrique M. Arpa, Miguel Gomez-Mendoza, Yuri Katayama, Victor A. de la Peña O’Shea, Céline Bouchel, Clément Jacob, Pierre-Georges Echeverria, Alessandro Ruffoni, Daniele Leonori
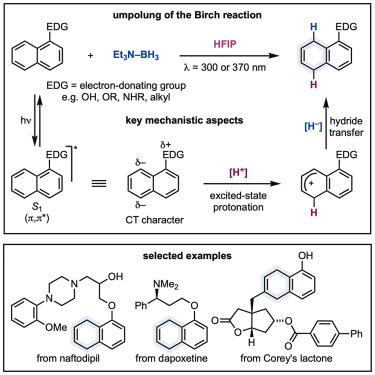
|
The Birch reaction is a classical process used for the partial reduction of aromatics into non-conjugated cyclohexadienes that can be further functionalized. This strategy and its more modern variants are all based on an initial single-electron transfer event converting the arene into the corresponding radical anion for either protonation or hydrogen-atom transfer. Herein, we demonstrate an umpolung approach where the aromatic is first protonated to its corresponding carbocation and then reduced using the Lewis acid-base complex Et3N−BH3. This strategy requires aromatic photoexcitation so that protonation is favored by charge-transfer and driven by excited-state antiaromaticity relief. This means that aromatic excited-state basicity rather than ground-state redox potential needs to be considered when approaching reaction development. The mild conditions and the avoidance of strong reductants have enabled tolerance of functionalities generally not compatible under standard Birch conditions.
中文翻译:

激发态质子化和还原使萘的 umpolung Birch 还原成为可能
Birch 反应是一种经典工艺,用于将芳烃部分还原成可以进一步功能化的非共轭环己二烯。这种策略及其更现代的变体都基于初始单电子转移事件,将芳烃转化为相应的自由基阴离子,用于质子化或氢原子转移。在这里,我们展示了一种 umpolung 方法,其中芳烃首先被质子化为其相应的碳阳离子,然后使用 Lewis 酸碱复合物 Et3N-BH3 进行还原。这种策略需要芳香族光激发,以便质子化受到电荷转移的青睐,并受到激发态反芳香性缓解的驱动。这意味着在进行反应开发时,需要考虑芳香族激发态碱度,而不是基态氧化还原电位。温和的条件和避免使用强还原剂,使得能够容忍在标准 Birch 条件下通常不兼容的功能。
更新日期:2024-11-22
中文翻译:

激发态质子化和还原使萘的 umpolung Birch 还原成为可能
Birch 反应是一种经典工艺,用于将芳烃部分还原成可以进一步功能化的非共轭环己二烯。这种策略及其更现代的变体都基于初始单电子转移事件,将芳烃转化为相应的自由基阴离子,用于质子化或氢原子转移。在这里,我们展示了一种 umpolung 方法,其中芳烃首先被质子化为其相应的碳阳离子,然后使用 Lewis 酸碱复合物 Et3N-BH3 进行还原。这种策略需要芳香族光激发,以便质子化受到电荷转移的青睐,并受到激发态反芳香性缓解的驱动。这意味着在进行反应开发时,需要考虑芳香族激发态碱度,而不是基态氧化还原电位。温和的条件和避免使用强还原剂,使得能够容忍在标准 Birch 条件下通常不兼容的功能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号