当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facet-dependent hematite reactivity in Cr(VI) removal with Fe(II)
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-22 , DOI: 10.1039/d4en00733f Shengnan Zhang, Lingyi Li, Junxue Li, Wei Cheng
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-22 , DOI: 10.1039/d4en00733f Shengnan Zhang, Lingyi Li, Junxue Li, Wei Cheng
|
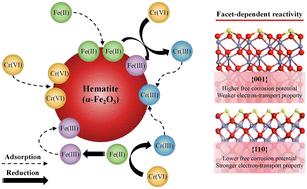
Hematite displays diverse crystal structures and often coexists with Fe(II), both of which are crucial in controlling the fate and mobility of Cr(VI). However, the mechanisms underlying Cr(VI) removal in the presence of Fe(II) on various hematite facets remain elusive. This study aims to elucidate the facet-dependent reactivity of hematite nanocrystals in conjunction with Fe(II) for the removal of Cr(VI) from aqueous solutions. Hematite nanoplates (HNPs), predominantly composed of {001} facets, and nanorods (HNRs), exposing both {001} and {110} facets, were synthesized and characterized. Their Cr(VI) removal capabilities were evaluated in hematite–Cr(VI) and hematite–Fe(II)–Cr(VI) systems, as well as the Fe(II)–Cr(VI) system. The adsorption of Fe(II) and Cr(VI) on hematite surfaces was highly dependent on the crystal facets and pH, with HNRs demonstrating superior Cr(VI) adsorption over HNPs, especially under acidic conditions. Neutral pH favored Fe(II)–Cr(VI) redox reactions and Fe(II) adsorption. The hematite–Fe(II) couple displayed a synergistic effect in removing Cr(VI) under acidic conditions, which was not observed under neutral conditions. The presence of Fe(II) notably enhanced Cr(VI) adsorption onto hematite, and bound Fe(II) facilitated electron transfer, accelerating Cr(VI) reduction. HNRs–Fe(II) exhibited higher Cr(VI) removal efficiency than HNPs–Fe(II) due to their lower free corrosion potential and improved electron transport properties. This research underscores the potential of facet engineering in optimizing hematite nanocrystals for environmental remediation, specifically in Cr(VI)-contaminated environments.
中文翻译:

使用 Fe(II) 去除 Cr(VI) 的刻面依赖性赤铁矿反应性
赤铁矿表现出多种晶体结构,并且经常与 Fe(II) 共存,这两者都对控制 Cr(VI) 的命运和迁移率至关重要。然而,在存在 Fe(II) 的情况下,各种赤铁矿刻面上 Cr(VI) 去除的机制仍然难以捉摸。本研究旨在阐明赤铁矿纳米晶体与 Fe(II) 结合从水溶液中去除 Cr(VI) 的刻面依赖性反应性。合成并表征了主要由 {001} 刻面组成的赤铁矿纳米板 (HNP) 和暴露{001}面和{110}面的纳米棒 (HNR)。在赤铁矿-Cr(VI)和赤铁矿-Fe(II)-Cr(VI)系统以及Fe(II)-Cr(VI)系统中评估了它们的Cr(VI)去除能力。Fe(II) 和 Cr(VI)在赤铁矿表面的吸附高度依赖于晶体刻面和 pH 值,HNR 表现出优于 HNP 的 Cr(VI)吸附,尤其是在酸性条件下。中性 pH 值有利于 Fe(II)–Cr(VI)氧化还原反应和 Fe(II) 吸附。赤铁矿-Fe(II) 对在酸性条件下表现出去除 Cr(VI) 的协同效应,这在中性条件下没有观察到。Fe(II) 的存在显著增强了 Cr(VI) 对赤铁矿的吸附,结合的 Fe(II) 促进了电子转移,加速了 Cr(VI) 的还原。HNRs-Fe(II) 表现出比 HNPs-Fe(II) 更高的 Cr(VI) 去除效率,因为它们的自由腐蚀电位较低且电子传输性能更好。 这项研究强调了刻面工程在优化赤铁矿纳米晶体以进行环境修复方面的潜力,特别是在 Cr(VI) 污染的环境中。
更新日期:2024-11-22
中文翻译:

使用 Fe(II) 去除 Cr(VI) 的刻面依赖性赤铁矿反应性
赤铁矿表现出多种晶体结构,并且经常与 Fe(II) 共存,这两者都对控制 Cr(VI) 的命运和迁移率至关重要。然而,在存在 Fe(II) 的情况下,各种赤铁矿刻面上 Cr(VI) 去除的机制仍然难以捉摸。本研究旨在阐明赤铁矿纳米晶体与 Fe(II) 结合从水溶液中去除 Cr(VI) 的刻面依赖性反应性。合成并表征了主要由 {001} 刻面组成的赤铁矿纳米板 (HNP) 和暴露{001}面和{110}面的纳米棒 (HNR)。在赤铁矿-Cr(VI)和赤铁矿-Fe(II)-Cr(VI)系统以及Fe(II)-Cr(VI)系统中评估了它们的Cr(VI)去除能力。Fe(II) 和 Cr(VI)在赤铁矿表面的吸附高度依赖于晶体刻面和 pH 值,HNR 表现出优于 HNP 的 Cr(VI)吸附,尤其是在酸性条件下。中性 pH 值有利于 Fe(II)–Cr(VI)氧化还原反应和 Fe(II) 吸附。赤铁矿-Fe(II) 对在酸性条件下表现出去除 Cr(VI) 的协同效应,这在中性条件下没有观察到。Fe(II) 的存在显著增强了 Cr(VI) 对赤铁矿的吸附,结合的 Fe(II) 促进了电子转移,加速了 Cr(VI) 的还原。HNRs-Fe(II) 表现出比 HNPs-Fe(II) 更高的 Cr(VI) 去除效率,因为它们的自由腐蚀电位较低且电子传输性能更好。 这项研究强调了刻面工程在优化赤铁矿纳米晶体以进行环境修复方面的潜力,特别是在 Cr(VI) 污染的环境中。






























 京公网安备 11010802027423号
京公网安备 11010802027423号