当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
S-Alkylation of sulfinamides with Zn-carbenoids: expanding stereoselective sulfoximine synthesis beyond NH derivatives
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-22 , DOI: 10.1039/d4qo01931h Glebs Jersovs, Dzonatans Melgalvis, Artis Kinens, Pavel A. Donets, Edgars Suna
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-22 , DOI: 10.1039/d4qo01931h Glebs Jersovs, Dzonatans Melgalvis, Artis Kinens, Pavel A. Donets, Edgars Suna
|
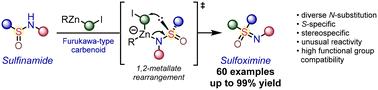
Sulfoximines are experiencing steadily increasing use in the development of pharmaceuticals and agrochemicals. Although recently a number of synthetic methods to access this versatile motif have been disclosed, only NH-sulfoximines have been considered as the ultimate targets. Here, we report an approach toward enantiopure N-substituted sulfoximines via direct stereoretentive S-alkylation of parent sulfinamides with zinc carbenoids. Mechanistically, a carbon–sulfur bond is formed in the course of 1,2-metallate rearrangement featuring an unusual migration of the S-atom in the transient zincate complex. The approach accommodates a large variety of differently substituted sulfinamides and features excellent functional group compatibility.
中文翻译:

亚磺酰胺与 Zn-carbenoids 的 S-烷基化反应:将立体选择性亚砜胺合成扩展到 NH 衍生物之外
砜肚在药物和农用化学品开发中的应用正在稳步增加。尽管最近已经披露了许多获得这种多功能基序的合成方法,但只有 NH-亚砜胺被认为是最终靶标。在这里,我们报道了一种通过母体亚磺酰胺与类锌碳烯基直接立体截留 S-烷基化来实现对映体纯 N 取代的砜胺的方法。从机制上讲,碳硫键是在 1,2 金属酸盐重排过程中形成的,其特征是 S 原子在瞬态锌酸盐配合物中发生不寻常的迁移。该方法适用于多种不同取代的亚磺酰胺,并具有出色的官能团相容性。
更新日期:2024-11-22
中文翻译:

亚磺酰胺与 Zn-carbenoids 的 S-烷基化反应:将立体选择性亚砜胺合成扩展到 NH 衍生物之外
砜肚在药物和农用化学品开发中的应用正在稳步增加。尽管最近已经披露了许多获得这种多功能基序的合成方法,但只有 NH-亚砜胺被认为是最终靶标。在这里,我们报道了一种通过母体亚磺酰胺与类锌碳烯基直接立体截留 S-烷基化来实现对映体纯 N 取代的砜胺的方法。从机制上讲,碳硫键是在 1,2 金属酸盐重排过程中形成的,其特征是 S 原子在瞬态锌酸盐配合物中发生不寻常的迁移。该方法适用于多种不同取代的亚磺酰胺,并具有出色的官能团相容性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号