Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer cells restrict immunogenicity of retrotransposon expression via distinct mechanisms
Immunity ( IF 25.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.immuni.2024.10.015 Siyu Sun, Eunae You, Jungeui Hong, David Hoyos, Isabella Del Priore, Kaloyan M. Tsanov, Om Mattagajasingh, Andrea Di Gioacchino, Sajid A. Marhon, Jonathan Chacon-Barahona, Hao Li, Hua Jiang, Samira Hozeifi, Omar Rosas-Bringas, Katherine H. Xu, Yuhui Song, Evan R. Lang, Alexandra S. Rojas, Linda T. Nieman, Bidish K. Patel, Rajmohan Murali, Pharto Chanda, Ali Karacay, Nicolas Vabret, Daniel D. De Carvalho, Daniel Zenklusen, John LaCava, Scott W. Lowe, David T. Ting, Christine A. Iacobuzio-Donahue, Alexander Solovyov, Benjamin D. Greenbaum
Immunity ( IF 25.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.immuni.2024.10.015 Siyu Sun, Eunae You, Jungeui Hong, David Hoyos, Isabella Del Priore, Kaloyan M. Tsanov, Om Mattagajasingh, Andrea Di Gioacchino, Sajid A. Marhon, Jonathan Chacon-Barahona, Hao Li, Hua Jiang, Samira Hozeifi, Omar Rosas-Bringas, Katherine H. Xu, Yuhui Song, Evan R. Lang, Alexandra S. Rojas, Linda T. Nieman, Bidish K. Patel, Rajmohan Murali, Pharto Chanda, Ali Karacay, Nicolas Vabret, Daniel D. De Carvalho, Daniel Zenklusen, John LaCava, Scott W. Lowe, David T. Ting, Christine A. Iacobuzio-Donahue, Alexander Solovyov, Benjamin D. Greenbaum
|
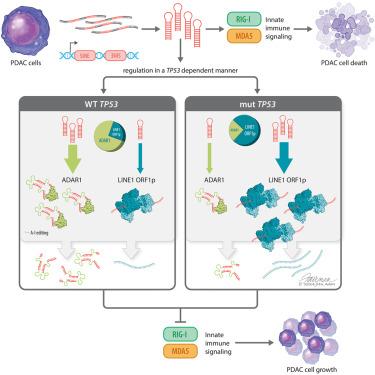
To thrive, cancer cells must navigate acute inflammatory signaling accompanying oncogenic transformation, such as via overexpression of repeat elements. We examined the relationship between immunostimulatory repeat expression, tumor evolution, and the tumor-immune microenvironment. Integration of multimodal data from a cohort of pancreatic ductal adenocarcinoma (PDAC) patients revealed expression of specific Alu repeats predicted to form double-stranded RNAs (dsRNAs) and trigger retinoic-acid-inducible gene I (RIG-I)-like-receptor (RLR)-associated type-I interferon (IFN) signaling. Such Alu-derived dsRNAs also anti-correlated with pro-tumorigenic macrophage infiltration in late stage tumors. We defined two complementary pathways whereby PDAC may adapt to such anti-tumorigenic signaling. In mutant TP53 tumors, ORF1p from long interspersed nuclear element (LINE)-1 preferentially binds Alus and decreases their expression, whereas adenosine deaminases acting on RNA 1 (ADAR1) editing primarily reduces dsRNA formation in wild-type TP53 tumors. Depletion of either LINE-1 ORF1p or ADAR1 reduced tumor growth in vitro . The fact that tumors utilize multiple pathways to mitigate immunostimulatory repeats implies the stress from their expression is a fundamental phenomenon to which PDAC, and likely other tumors, adapt.
中文翻译:

癌细胞通过不同的机制限制反转录转座子表达的免疫原性
为了茁壮成长,癌细胞必须通过伴随致癌转化的急性炎症信号传导,例如通过重复元件的过表达。我们检查了免疫刺激重复表达、肿瘤进化和肿瘤免疫微环境之间的关系。来自胰腺导管腺癌 (PDAC) 患者队列的多模式数据的整合揭示了预测形成双链 RNA (dsRNA) 并触发视黄酸诱导基因 I (RIG-I) 样受体 (RLR) 相关的 I 型干扰素 (IFN) 信号传导的特异性 Alu 重复序列的表达。这种 Alu 来源的 dsRNAs 也与晚期肿瘤中的促肿瘤巨噬细胞浸润呈反相关。我们定义了两种互补途径,PDAC 可能通过这些途径适应这种抗肿瘤信号传导。在突变的 TP53 肿瘤中,来自长散布核元件 (LINE)-1 的 ORF1p 优先结合 Alus 并降低其表达,而作用于 RNA 1 (ADAR1) 编辑的腺苷脱氨酶主要减少野生型 TP53 肿瘤中 dsRNA 的形成。LINE-1 ORF1p 或 ADAR1 的耗竭减少了体外肿瘤生长 。肿瘤利用多种途径来减轻免疫刺激重复的事实意味着其表达产生的压力是 PDAC 以及可能的其他肿瘤适应的基本现象。
更新日期:2024-11-21
中文翻译:

癌细胞通过不同的机制限制反转录转座子表达的免疫原性
为了茁壮成长,癌细胞必须通过伴随致癌转化的急性炎症信号传导,例如通过重复元件的过表达。我们检查了免疫刺激重复表达、肿瘤进化和肿瘤免疫微环境之间的关系。来自胰腺导管腺癌 (PDAC) 患者队列的多模式数据的整合揭示了预测形成双链 RNA (dsRNA) 并触发视黄酸诱导基因 I (RIG-I) 样受体 (RLR) 相关的 I 型干扰素 (IFN) 信号传导的特异性 Alu 重复序列的表达。这种 Alu 来源的 dsRNAs 也与晚期肿瘤中的促肿瘤巨噬细胞浸润呈反相关。我们定义了两种互补途径,PDAC 可能通过这些途径适应这种抗肿瘤信号传导。在突变的 TP53 肿瘤中,来自长散布核元件 (LINE)-1 的 ORF1p 优先结合 Alus 并降低其表达,而作用于 RNA 1 (ADAR1) 编辑的腺苷脱氨酶主要减少野生型 TP53 肿瘤中 dsRNA 的形成。LINE-1 ORF1p 或 ADAR1 的耗竭减少了体外肿瘤生长 。肿瘤利用多种途径来减轻免疫刺激重复的事实意味着其表达产生的压力是 PDAC 以及可能的其他肿瘤适应的基本现象。






























 京公网安备 11010802027423号
京公网安备 11010802027423号