当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into the electrochemical oxidation of acetate at noble metals
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.checat.2024.101190 Venkata Sai Sriram Mosali, Hanna Soucie, Xiong Peng, Ehsan Faegh, Matthew Elam, Ian Street, William E. Mustain
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.checat.2024.101190 Venkata Sai Sriram Mosali, Hanna Soucie, Xiong Peng, Ehsan Faegh, Matthew Elam, Ian Street, William E. Mustain
|
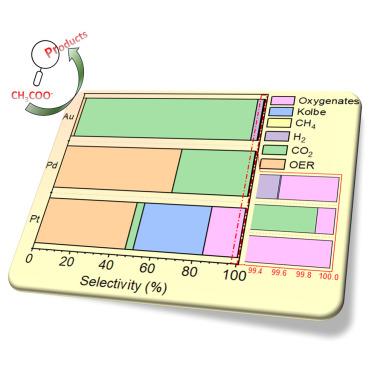
Electrochemical acetate oxidation (AcOR) offers a sustainable approach to produce renewable biofuels. While CO₂ formation is thermodynamically favored, acetate oxidation can also yield various products through the Kolbe and Hofer-Moest mechanisms, enabling a modulation of the products formed via partial oxidation. Given the complexity of the reaction, it is crucial to understand how different reaction conditions influence the product profile. Furthermore, this process generates methyl radicals, providing insights into methane partial oxidation. The current study explores AcOR on noble metal electrodes (Pt, Pd, Au) in a 0.5 M CH3COOK aqueous electrolyte, revealing the mechanism of product formation using potential- and time-dependent electrolysis and isotope-labeling experiments. The effect of surface chemistry, ion transport, electrolyte concentration, and electrolysis techniques on product selectivity is analyzed. Additionally, the study compares product profiles from an electrolyzer cell to those obtained from model electrodes in batch-cell setup.
中文翻译:

贵金属中乙酸盐电化学氧化的机理见解
电化学乙酸盐氧化 (AcOR) 提供了一种生产可再生生物燃料的可持续方法。虽然热力学上有利于 CO₂ 的形成,但乙酸盐氧化也可以通过 Kolbe 和 Hofer-Moest 机制产生各种产物,从而能够调节通过部分氧化形成的产物。鉴于反应的复杂性,了解不同的反应条件如何影响产品特性至关重要。此外,这个过程会产生甲基自由基,从而提供对甲烷部分氧化的见解。目前的研究探索了 0.5 M CH3COOK 水性电解质中贵金属电极(Pt、Pd、Au)上的 AcOR,使用电位和时间依赖性电解和同位素标记实验揭示了产物形成的机制。分析了表面化学、离子传输、电解质浓度和电解技术对产品选择性的影响。此外,该研究还比较了电解槽的产品曲线与批处理池设置中从模型电极获得的产品曲线。
更新日期:2024-11-21
中文翻译:

贵金属中乙酸盐电化学氧化的机理见解
电化学乙酸盐氧化 (AcOR) 提供了一种生产可再生生物燃料的可持续方法。虽然热力学上有利于 CO₂ 的形成,但乙酸盐氧化也可以通过 Kolbe 和 Hofer-Moest 机制产生各种产物,从而能够调节通过部分氧化形成的产物。鉴于反应的复杂性,了解不同的反应条件如何影响产品特性至关重要。此外,这个过程会产生甲基自由基,从而提供对甲烷部分氧化的见解。目前的研究探索了 0.5 M CH3COOK 水性电解质中贵金属电极(Pt、Pd、Au)上的 AcOR,使用电位和时间依赖性电解和同位素标记实验揭示了产物形成的机制。分析了表面化学、离子传输、电解质浓度和电解技术对产品选择性的影响。此外,该研究还比较了电解槽的产品曲线与批处理池设置中从模型电极获得的产品曲线。






























 京公网安备 11010802027423号
京公网安备 11010802027423号