Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to cyclobutane–fused dihydrobenzothiophenes via gold–mediated photocatalyzed [2+2]–cycloaddition reactions
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.jcat.2024.115850 Ekaterina A. Martynova, Vladislav V. Voloshkin, Marco Villa, Antonio Fiorentino, Marek Beliš, Kristof Van Hecke, Paola Ceroni, Steven P. Nolan
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.jcat.2024.115850 Ekaterina A. Martynova, Vladislav V. Voloshkin, Marco Villa, Antonio Fiorentino, Marek Beliš, Kristof Van Hecke, Paola Ceroni, Steven P. Nolan
|
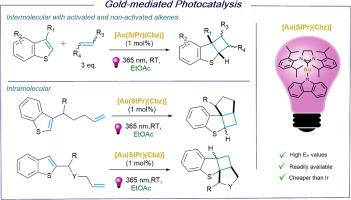
The use of [Au(SIPr)(Cbz)] as photosensitizer in [2+2]–cycloaddition reactions between benzothiophenes and activated and non–activated alkenes is presented. Commonly used organic and Ir–photosensitizers proved inefficient in the reaction with non–activated alkenes. The study emphasizes the dependence on the nature of the alkene, a parameter that has previously received little attention. Under mild reaction conditions, a wide array of alkenes and benzothiophenes were successfully coupled, showcasing remarkable tolerance of diverse functional groups. Additionally, we present intramolecular [2+2]–cycloaddition reactions, including benzothiophenes featuring various alkene linkers at the C2 and C3 positions.
中文翻译:

通过金介导的光催化 [2+2] -环加成反应获得环丁烷-熔融二氢苯并噻吩
介绍了在苯并噻吩与活化和非活化烯烃之间的 [2+2] -环加成反应中使用 [Au(SIPr)(Cbz)] 作为光敏剂。常用的有机和 Ir-光敏剂在与非活化烯烃的反应中被证明效率低下。该研究强调了对烯烃性质的依赖性,这一参数以前很少受到关注。在温和的反应条件下,多种烯烃和苯并噻吩成功偶联,显示出对不同官能团的显著耐受性。此外,我们还提出了分子内 [2+2] - 环加成反应,包括在 C2 和 C3 位置具有各种烯烃接头的苯并噻吩。
更新日期:2024-11-21
中文翻译:

通过金介导的光催化 [2+2] -环加成反应获得环丁烷-熔融二氢苯并噻吩
介绍了在苯并噻吩与活化和非活化烯烃之间的 [2+2] -环加成反应中使用 [Au(SIPr)(Cbz)] 作为光敏剂。常用的有机和 Ir-光敏剂在与非活化烯烃的反应中被证明效率低下。该研究强调了对烯烃性质的依赖性,这一参数以前很少受到关注。在温和的反应条件下,多种烯烃和苯并噻吩成功偶联,显示出对不同官能团的显著耐受性。此外,我们还提出了分子内 [2+2] - 环加成反应,包括在 C2 和 C3 位置具有各种烯烃接头的苯并噻吩。






























 京公网安备 11010802027423号
京公网安备 11010802027423号