当前位置:
X-MOL 学术
›
Mater. Today Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of bifunctional MOF-based composite electrocatalysts promoting oxygen evolution reaction and glucose oxidation reaction and its kinetic deciphering
Materials Today Physics ( IF 10.0 ) Pub Date : 2024-11-20 , DOI: 10.1016/j.mtphys.2024.101601 Hongmei Yuan, Changyu Weng, Xinghua Zhang, Lungang Chen, Qi Zhang, Longlong Ma, Jianguo Liu
Materials Today Physics ( IF 10.0 ) Pub Date : 2024-11-20 , DOI: 10.1016/j.mtphys.2024.101601 Hongmei Yuan, Changyu Weng, Xinghua Zhang, Lungang Chen, Qi Zhang, Longlong Ma, Jianguo Liu
|
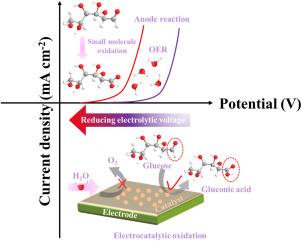
The climate crisis and the need for green and sustainable energy drive the rapid development of hydrogen production from water electrolysis. Improvements in the kinetics of the anode reaction, which governs the efficiency of water electrolysis, are essential for efficient hydrogen production and key to effectively addressing global environmental and energy challenges. Hence, we focus on improving the kinetics of the anode oxidation reaction. The multi-walled carbon nanotubes coupled with bimetallic organic framework (CoFe-MOF-74) composite electrocatalysts (CoFe-MOF-74@MWCNT) were fabricated for OER and the kinetically more favorable glucose oxidation reaction (GOR). Compared to commercial RuO2 , CoFe-MOF-74@MWCNT showed superior OER catalytic performance, exhibiting a lower overpotential (273 mV) and a lower Tafel slope (55 mV dec−1 ) at a current density of 10 mA cm−2 . Moreover, after adding glucose to the anode, the potential required of 10 mA cm−2 was only 1.291 V (vs. RHE), a reduction of 212 mV compared to the OER potential. This reduction in potential demonstrates the efficiency of our catalysts and signifies significant energy savings. The characterization results and theoretical calculations indicated that the superior OER/GOR performance of CoFe-MOF-74@MWCNT can be ascribed to the synergistic effect between MWCNT and the mixed metal nodes of the bimetallic organic framework. The doping of MWCNT promoted the catalyst charge transfer efficiency (Rct was only 5.56 Ω) in the OER process. The mixed metal nodes of CoFe-MOF-74@MWCNT provided more active sites for the electrocatalytic reaction, and promoted the bond-breaking of critical intermediates in the oxidation process, significantly reducing the free energy of catalytic intermediates and accelerating reaction kinetics. This work provides a strategy for designing multifunctional electrocatalysts for OER and biomass small molecule oxidation and highlights the potential for significant energy savings in practical applications.
中文翻译:

促进析氧反应和葡萄糖氧化反应的双功能MOF基复合电催化剂的构建及其动力学破译
气候危机和对绿色和可持续能源的需求推动了电解水制氢的快速发展。阳极反应的动力学控制着水电解的效率,其动力学的改进对于高效制氢至关重要,也是有效应对全球环境和能源挑战的关键。因此,我们专注于改善阳极氧化反应的动力学。制备了多壁碳纳米管与双金属有机框架 (CoFe-MOF-74) 复合电催化剂 (CoFe-MOF-74@MWCNT) 耦合,用于 OER 和动力学上更有利的葡萄糖氧化反应 (GOR)。与商用 RuO2 相比,CoFe-MOF-74@MWCNT 表现出卓越的 OER 催化性能,在 10 mA cm-2 的电流密度下表现出较低的过电位 (273 mV) 和较低的塔菲尔斜率 (55 mV dec-1)。此外,在阳极中添加葡萄糖后,所需的 10 mA cm-2 电位仅为 1.291 V(与 RHE 相比),与 OER 电位相比降低了 212 mV。这种电位的降低证明了我们催化剂的效率,并意味着显著的能源节约。表征结果和理论计算表明,CoFe-MOF-74@MWCNT 优异的 OER/GOR 性能可归因于 MWCNT 与双金属有机框架的混合金属节点之间的协同作用。MWCNT 的掺杂提高了 OER 过程中催化剂电荷转移效率 (Rct 仅为 5.56 Ω)。 CoFe-MOF-74@MWCNT 的混合金属节点为电催化反应提供了更多的活性位点,并促进了氧化过程中关键中间体的键断裂,显著降低了催化中间体的自由能,加速了反应动力学。这项工作为设计用于 OER 和生物质小分子氧化的多功能电催化剂提供了一种策略,并强调了在实际应用中显著节能的潜力。
更新日期:2024-11-20
中文翻译:

促进析氧反应和葡萄糖氧化反应的双功能MOF基复合电催化剂的构建及其动力学破译
气候危机和对绿色和可持续能源的需求推动了电解水制氢的快速发展。阳极反应的动力学控制着水电解的效率,其动力学的改进对于高效制氢至关重要,也是有效应对全球环境和能源挑战的关键。因此,我们专注于改善阳极氧化反应的动力学。制备了多壁碳纳米管与双金属有机框架 (CoFe-MOF-74) 复合电催化剂 (CoFe-MOF-74@MWCNT) 耦合,用于 OER 和动力学上更有利的葡萄糖氧化反应 (GOR)。与商用 RuO2 相比,CoFe-MOF-74@MWCNT 表现出卓越的 OER 催化性能,在 10 mA cm-2 的电流密度下表现出较低的过电位 (273 mV) 和较低的塔菲尔斜率 (55 mV dec-1)。此外,在阳极中添加葡萄糖后,所需的 10 mA cm-2 电位仅为 1.291 V(与 RHE 相比),与 OER 电位相比降低了 212 mV。这种电位的降低证明了我们催化剂的效率,并意味着显著的能源节约。表征结果和理论计算表明,CoFe-MOF-74@MWCNT 优异的 OER/GOR 性能可归因于 MWCNT 与双金属有机框架的混合金属节点之间的协同作用。MWCNT 的掺杂提高了 OER 过程中催化剂电荷转移效率 (Rct 仅为 5.56 Ω)。 CoFe-MOF-74@MWCNT 的混合金属节点为电催化反应提供了更多的活性位点,并促进了氧化过程中关键中间体的键断裂,显著降低了催化中间体的自由能,加速了反应动力学。这项工作为设计用于 OER 和生物质小分子氧化的多功能电催化剂提供了一种策略,并强调了在实际应用中显著节能的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号