当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Mechanism of Biomimetic Arene Hydroxylation: When a Diiron Metal Center Meets a Sulfur-Containing Ligand
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acscatal.4c04662 Ya-Ru Sheng, Bo Bi, Lu Cheng, Wei Han, Hui Chen
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acscatal.4c04662 Ya-Ru Sheng, Bo Bi, Lu Cheng, Wei Han, Hui Chen
|
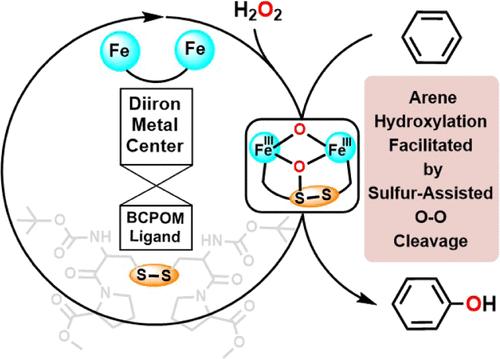
Efficient and selective arene hydroxylation under mild reaction conditions is a challenging task in chemical transformation. To achieve this goal, one of us recently reported an experimental breakthrough of a highly efficient iron catalyst based on the sulfur-containing ligand BCPOM. However, the exact mechanism underlying this promising biomimetic catalysis remained elusive. Herein, based on density functional theory modelings combined with experimental results, we successfully revealed an unexpected mechanism of this biomimetic arene hydroxylation. In this mechanism of diiron/BCPOM, the disulfide group of the ligand was found to assist the O–O cleavage of the peroxo species by concomitantly forming an S–O bond, which thus generated an uncommon diferric diiron-oxo intermediate as the real oxidant for the subsequent arene hydroxylation. In this way, the revealed hydroxylation mechanism with diiron/BCPOM differs not only from the mononuclear heme enzyme P450 but also from the diiron nonheme enzyme T4MO substantially. Consistent with the NIH shift experimental results, this mechanism also enabled the experimentally confirmed regioselectivity prediction for some substrates unexplored previously. The unexpected role played by the sulfur-containing ligand in assisting the O–O cleavage by forming the S–O bond further expands our knowledge on how sulfur can facilitate the iron-catalyzed reactions.
中文翻译:

探究仿生 Arene 羟基化的机理:当 Diiron 金属中心遇到含硫配体时
在温和的反应条件下,高效和选择性的芳烃羟基化是化学转化中一项具有挑战性的任务。为了实现这一目标,我们中的一位最近报道了一种基于含硫配体 BCPOM 的高效铁催化剂的实验突破。然而,这种有前途的仿生催化背后的确切机制仍然难以捉摸。在此,基于密度泛函理论建模结合实验结果,我们成功地揭示了这种仿生芳烃羟基化的意想不到的机制。在这种二铁/BCPOM 机制中,发现配体的二硫键通过同时形成 S-O 键来帮助 peroxo 物种的 O-O 裂解,从而产生不常见的二铁二铁-氧基化合物中间体作为随后芳烃羟基化的真正氧化剂。通过这种方式,揭示的二铁/BCPOM 羟基化机制不仅与单核血红素酶 P450 不同,而且与二铁非血红素酶 T4MO 也大不相同。与 NIH 偏移实验结果一致,这种机制还能够对一些以前未探索的底物进行实验证实的区域选择性预测。含硫配体通过形成 S-O 键在协助 O-O 裂解中发挥的意想不到的作用进一步扩展了我们对硫如何促进铁催化反应的了解。
更新日期:2024-11-20
中文翻译:

探究仿生 Arene 羟基化的机理:当 Diiron 金属中心遇到含硫配体时
在温和的反应条件下,高效和选择性的芳烃羟基化是化学转化中一项具有挑战性的任务。为了实现这一目标,我们中的一位最近报道了一种基于含硫配体 BCPOM 的高效铁催化剂的实验突破。然而,这种有前途的仿生催化背后的确切机制仍然难以捉摸。在此,基于密度泛函理论建模结合实验结果,我们成功地揭示了这种仿生芳烃羟基化的意想不到的机制。在这种二铁/BCPOM 机制中,发现配体的二硫键通过同时形成 S-O 键来帮助 peroxo 物种的 O-O 裂解,从而产生不常见的二铁二铁-氧基化合物中间体作为随后芳烃羟基化的真正氧化剂。通过这种方式,揭示的二铁/BCPOM 羟基化机制不仅与单核血红素酶 P450 不同,而且与二铁非血红素酶 T4MO 也大不相同。与 NIH 偏移实验结果一致,这种机制还能够对一些以前未探索的底物进行实验证实的区域选择性预测。含硫配体通过形成 S-O 键在协助 O-O 裂解中发挥的意想不到的作用进一步扩展了我们对硫如何促进铁催化反应的了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号