当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Reaction Network of Ethylene Epoxidation on Partially Oxidized Silver Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acscatal.4c04521 Adhika Setiawan, Tiancheng Pu, Israel E. Wachs, Srinivas Rangarajan
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acscatal.4c04521 Adhika Setiawan, Tiancheng Pu, Israel E. Wachs, Srinivas Rangarajan
|
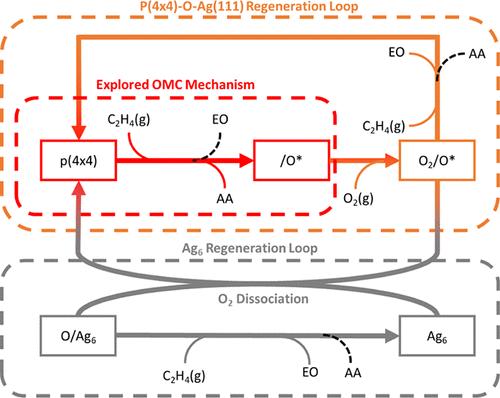
An extended microkinetic model (MKM) for the selective oxidation of ethylene to ethylene oxide (EO) is presented, based on an oxidic representation of the silver (Ag) surface, namely, the p(4 × 4) oxidic reconstruction of the Ag(111) phase to mimic the significant oxygen coverage under reaction conditions, as is evidenced by recent operando spectroscopic studies. The MKM features three pathways each for producing either ethylene oxide (EO) or carbon dioxide (CO2), including the common intermediate or oxometallacycle (OMC) pathway, an atomic oxygen pathway, as well as pathways centered around the role of a diatomic oxygen species occupying an oxygen vacancy (O2/O*). The MKM uses a composite set of experimental and density functional theory (DFT) kinetic parameters, which is further optimized and trained on experimental reaction data. A multistart ensemble approach was used to ensure a thorough sampling of the solution space, and a closer analysis was performed on the best-performing, physically meaningful solution. In agreement with published DFT data, the optimized MKM observed that the OMC pathway heavily favors the total combustion pathway and alone is insufficient in explaining the ∼50% EO selectivity commonly reported. Furthermore, it confirmed the pivotal role of the O2/O* species in the flux-carrying pathways for EO production. The MKM additionally highlights the fluctuating nature of the catalyst surface, in that the proportion of metallic to oxidic phase changes according to the reaction conditions, accordingly resulting in kinetic implications.
中文翻译:

扩展乙烯环氧化对部分氧化银催化剂的反应网络
提出了一种用于乙烯选择性氧化为环氧乙烷 (EO) 的扩展微动力学模型 (MKM),基于银 (Ag) 表面的氧化表示,即 Ag(111) 相的 p(4 × 4) 氧化重建,以模拟反应条件下显着的氧覆盖率,最近的原位光谱研究证明了这一点。MKM 具有三个途径,分别用于产生环氧乙烷 (EO) 或二氧化碳 (CO2),包括共同中间体或氧金属循环 (OMC) 途径、原子氧途径,以及以占据氧空位 (O2/O*) 的双原子氧物质的作用为中心的途径。MKM 使用一组复合的实验和密度泛函理论 (DFT) 动力学参数,这些参数在实验反应数据上进行了进一步优化和训练。使用多起点集成方法确保对解空间进行全面采样,并对性能最佳、具有物理意义的解决方案进行更仔细的分析。与已发表的 DFT 数据一致,优化的 MKM 观察到 OMC 途径严重偏向于总燃烧途径,仅凭这一点不足以解释通常报道的 ∼50% EO 选择性。此外,它证实了 O2/O* 物质在 EO 产生的助焊剂携带途径中的关键作用。MKM 还强调了催化剂表面的波动性,因为金属相与氧化相的比例根据反应条件而变化,从而产生动力学影响。
更新日期:2024-11-20
中文翻译:

扩展乙烯环氧化对部分氧化银催化剂的反应网络
提出了一种用于乙烯选择性氧化为环氧乙烷 (EO) 的扩展微动力学模型 (MKM),基于银 (Ag) 表面的氧化表示,即 Ag(111) 相的 p(4 × 4) 氧化重建,以模拟反应条件下显着的氧覆盖率,最近的原位光谱研究证明了这一点。MKM 具有三个途径,分别用于产生环氧乙烷 (EO) 或二氧化碳 (CO2),包括共同中间体或氧金属循环 (OMC) 途径、原子氧途径,以及以占据氧空位 (O2/O*) 的双原子氧物质的作用为中心的途径。MKM 使用一组复合的实验和密度泛函理论 (DFT) 动力学参数,这些参数在实验反应数据上进行了进一步优化和训练。使用多起点集成方法确保对解空间进行全面采样,并对性能最佳、具有物理意义的解决方案进行更仔细的分析。与已发表的 DFT 数据一致,优化的 MKM 观察到 OMC 途径严重偏向于总燃烧途径,仅凭这一点不足以解释通常报道的 ∼50% EO 选择性。此外,它证实了 O2/O* 物质在 EO 产生的助焊剂携带途径中的关键作用。MKM 还强调了催化剂表面的波动性,因为金属相与氧化相的比例根据反应条件而变化,从而产生动力学影响。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号