当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoted Electrochemical Ammonia Synthesis from Nitrate at the Ag–Cu Biphasic Interface
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acscatal.4c05465 Xinyang Gao, Chenyuan Zhu, Chunlei Yang, Guoshuai Shi, Qinshang Xu, Liming Zhang
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acscatal.4c05465 Xinyang Gao, Chenyuan Zhu, Chunlei Yang, Guoshuai Shi, Qinshang Xu, Liming Zhang
|
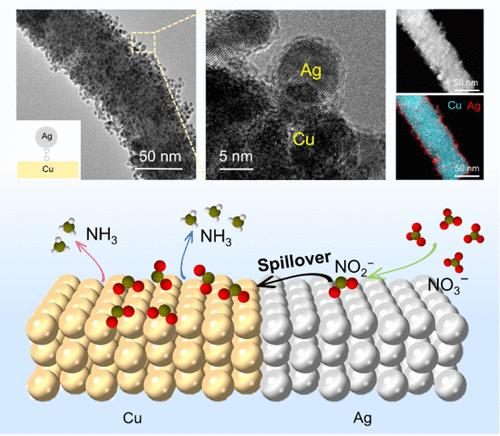
Electrochemical nitrate reduction (NO3–R) presents a promising pathway for carbon-neutral ammonia (NH3) synthesis. Enhancing NH3 selectivity through a tandem process can be achieved by combining Cu with a secondary metal, which allows for an adjustable binding energy between the bimetallic catalyst and key nitrogen intermediates. Herein, we developed a biphasic Ag–Cu heterostructure with a controllable elemental composition, which significantly improved NH3 production through tandem NO3–R. In-situ infrared spectroscopy and finite element simulations revealed that Ag serves as the active site for converting NO3– to NO2–, leading to a high localized concentration of NO2–, which is subsequently reduced to NH3 on adjacent Cu sites. Density functional theory calculations further confirmed the critical role of the Ag–Cu biphasic interface in promoting tandem NH3 production. This work offers valuable insights into the tandem NO3–R pathway in bimetallic heterostructures, providing a foundation for optimizing catalysts and advancing large-scale sustainable NH3 synthesis.
中文翻译:

促进在 Ag-Cu 双相界面处从硝酸盐电化学合成氨
电化学硝酸盐还原 (NO3–R) 为碳中和氨 (NH3) 合成提供了一种有前途的途径。通过将 Cu 与仲金属结合,可以通过串联工艺提高 NH3 选择性,这允许双金属催化剂和关键氮中间体之间的结合能可调。在此,我们开发了一种具有可控元素组成的双相 Ag-Cu 异质结构,通过串联 NO 3-R 显着改善了 NH3 的产生。原位红外光谱和有限元模拟表明,Ag 是将 NO3– 转化为 NO2– 的活性位点,导致 NO2– 的高局部浓度,随后被还原为 NH3在相邻的 Cu 位点上。密度泛函理论计算进一步证实了 Ag-Cu 双相界面在促进串联 NH3 产生中的关键作用。这项工作为双金属异质结构中的串联 NO 3-R 途径提供了有价值的见解,为优化催化剂和推进大规模可持续 NH3 合成提供了基础。
更新日期:2024-11-20
中文翻译:

促进在 Ag-Cu 双相界面处从硝酸盐电化学合成氨
电化学硝酸盐还原 (NO3–R) 为碳中和氨 (NH3) 合成提供了一种有前途的途径。通过将 Cu 与仲金属结合,可以通过串联工艺提高 NH3 选择性,这允许双金属催化剂和关键氮中间体之间的结合能可调。在此,我们开发了一种具有可控元素组成的双相 Ag-Cu 异质结构,通过串联 NO 3-R 显着改善了 NH3 的产生。原位红外光谱和有限元模拟表明,Ag 是将 NO3– 转化为 NO2– 的活性位点,导致 NO2– 的高局部浓度,随后被还原为 NH3在相邻的 Cu 位点上。密度泛函理论计算进一步证实了 Ag-Cu 双相界面在促进串联 NH3 产生中的关键作用。这项工作为双金属异质结构中的串联 NO 3-R 途径提供了有价值的见解,为优化催化剂和推进大规模可持续 NH3 合成提供了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号