当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of β-AlF3 from fluorine-containing wastes: Leaching with fluorosilicic acid and crystallization
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-20 , DOI: 10.1016/j.seppur.2024.130618 Xiaojun Lv, Xuan Tan, Zexun Han, Yongcong Wu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-20 , DOI: 10.1016/j.seppur.2024.130618 Xiaojun Lv, Xuan Tan, Zexun Han, Yongcong Wu
|
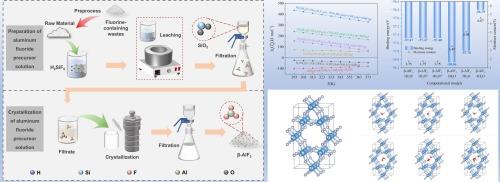
Numerous fluorine-containing hazardous solid wastes from the electrolytic aluminum process pose a serious threat to the ecosystem and human health. Currently, in the study of leaching on these wastes, Al and F are usually recovered by adjusting the pH of the leaching solution to precipitate aluminium hydroxyfluoride hydrate, which can be used to produce aluminum fluoride by roasting. However, aluminium hydroxyfluoride hydrate is often mingled with cryolite and other impurities when precipitating, which ultimately affects the purity of obtained aluminum fluoride by calcination. Interestingly, herein, the aluminium hydroxyfluoride hydrate residue was digested by fluorosilicic acid to effectively remove impurities and obtain a pure aluminum fluoride solution, from which the β-AlF3 product was produced by crystallization at a high-temperature. The results show that under the conditions of a temperature of 60 °C, time of 35 min, initial fluorine-aluminum molar ratio of 3:1 and initial concentration of fluorosilicic acid of 18 %, the gross yield of fluorine is 86.2 %, and the recovery of silicon in the form of SiO2 is 95.2 %. During crystallization, the product changes from AlF3 ·3H2 O to β-AlF3 with the increase of temperature. Under the conditions of a crystallization temperature of 150 °C, an initial concentration of aluminum fluoride of 191.10 g/L and a stirring speed of 200 rpm, β-AlF3 of an average particle size of 43.22 μm was obtained by adding 5 % seed. The contents of Al and F in β-AlF3 products are 32.57 % and 61.49 % respectively, which meet the requirements of GB/T 4292–2017 (AF-0) about National Standards of China. According to DFT calculation, the β-AlF3 tends to adsorb two or three water molecules in its cavity structure, which explains why the crystallized β-AlF3 contains water of 5.10 % at 180 °C.
更新日期:2024-11-20




















































 京公网安备 11010802027423号
京公网安备 11010802027423号