当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective separation of Co(II) from leachate of lithium-ion battery cathode material using novel molecular sieve-based MOFs composite NaA-MOFs(Al)
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130560 Chuang Chen, Yue Wang, Qi Zou, Liting Ding, Letian Ji, Jiayi Lu, Yaling Song, Wei Xiong, Guoyuan Yuan
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130560 Chuang Chen, Yue Wang, Qi Zou, Liting Ding, Letian Ji, Jiayi Lu, Yaling Song, Wei Xiong, Guoyuan Yuan
|
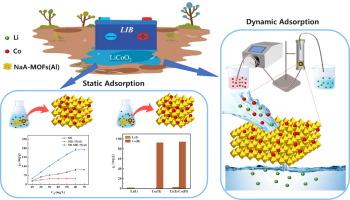
As the electric vehicle sector experiences swift expansion, there is a notable surge in the quantity of depleted lithium-ion batteries (LIBs). Effective management of these batteries involves separating key metals to enable resource recovery and reuse. The leachate from LIBs (L-LIBs) primarily contains Co(II) and Li(I), and selective extraction of Co(II) is crucial for reducing resource waste and promoting sustainability. In this study, a cost-effective and structurally stable Na-based type A molecular sieve (NaA) MOFs composite, NaA-MOFs(Al), was compounded through a straightforward two-step method for the selective extraction of Co(II) from L-LIBs. The results from the static adsorption trials indicate that the NaA-MOFs(Al) achieves a theoretical saturation point of 435.7 mg/g in its Co(II) adsorptive capacity, surpassing all previously documented values. The material achieved monolayer chemical adsorption through coordination interactions and exhibited excellent selectivity with a Li(I) selectivity coefficient of 328.4. Dynamic separation experiments further demonstrated that NaA-MOFs(Al) could completely separate Co(II) and Li(I), exhibiting a dynamic adsorption capacity of 512.0 mg/g, which aligns closely with the theoretical expectations from the Thomas model. The successful preparation of this new composite material not only reduces the cost of adsorbents but also significantly improves the adsorption capacity and selectivity for Co(II). This advancement provides great potential for the efficient recycling of lithium batteries and contributes to the sustainable development of the new energy industry.
中文翻译:

使用新型分子筛基 MOFs 复合 NaA-MOFs(Al) 从锂离子电池正极材料渗滤液中选择性分离 Co(II)
随着电动汽车行业的迅速扩张,耗尽的锂离子电池 (LIB) 数量显着激增。对这些电池的有效管理包括分离关键金属以实现资源回收和再利用。LIBs (L-LIBs) 的渗滤液主要含有 Co(II) 和 Li(I),选择性提取 Co(II) 对于减少资源浪费和促进可持续性至关重要。在本研究中,通过简单的两步法从 L-LIBs 中选择性提取 Co(II),合成了一种经济高效且结构稳定的 Na 基 A 型分子筛 (NaA) MOF 复合材料 NaA-MOFs(Al)。静态吸附试验的结果表明,NaA-MOFs(Al) 的 Co(II) 吸附容量达到 435.7 mg/g 的理论饱和点,超过了以前记录的所有值。该材料通过配位相互作用实现了单层化学吸附,并表现出优异的选择性,Li(I) 选择性系数为 328.4。动态分离实验进一步表明,NaA-MOFs(Al) 可以完全分离 Co(II) 和 Li(I),动态吸附容量为 512.0 mg/g,这与 Thomas 模型的理论预期非常吻合。这种新型复合材料的成功制备不仅降低了吸附剂的成本,而且显著提高了对 Co(II) 的吸附容量和选择性。这一进步为锂电池的高效回收提供了巨大潜力,为新能源行业的可持续发展做出了贡献。
更新日期:2024-11-20
中文翻译:

使用新型分子筛基 MOFs 复合 NaA-MOFs(Al) 从锂离子电池正极材料渗滤液中选择性分离 Co(II)
随着电动汽车行业的迅速扩张,耗尽的锂离子电池 (LIB) 数量显着激增。对这些电池的有效管理包括分离关键金属以实现资源回收和再利用。LIBs (L-LIBs) 的渗滤液主要含有 Co(II) 和 Li(I),选择性提取 Co(II) 对于减少资源浪费和促进可持续性至关重要。在本研究中,通过简单的两步法从 L-LIBs 中选择性提取 Co(II),合成了一种经济高效且结构稳定的 Na 基 A 型分子筛 (NaA) MOF 复合材料 NaA-MOFs(Al)。静态吸附试验的结果表明,NaA-MOFs(Al) 的 Co(II) 吸附容量达到 435.7 mg/g 的理论饱和点,超过了以前记录的所有值。该材料通过配位相互作用实现了单层化学吸附,并表现出优异的选择性,Li(I) 选择性系数为 328.4。动态分离实验进一步表明,NaA-MOFs(Al) 可以完全分离 Co(II) 和 Li(I),动态吸附容量为 512.0 mg/g,这与 Thomas 模型的理论预期非常吻合。这种新型复合材料的成功制备不仅降低了吸附剂的成本,而且显著提高了对 Co(II) 的吸附容量和选择性。这一进步为锂电池的高效回收提供了巨大潜力,为新能源行业的可持续发展做出了贡献。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号