当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Intramolecular Enantioselective Reductive Heck Reaction toward the Synthesis of Chiral 3-Trifluoromethylated Oxindoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.joc.4c01488 Qiang Wang, Zi-Sheng Ruan, Hong-Peng Wang, Ren-Xiao Liang, Yuan-Yuan Hu, Yi-Xia Jia
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.joc.4c01488 Qiang Wang, Zi-Sheng Ruan, Hong-Peng Wang, Ren-Xiao Liang, Yuan-Yuan Hu, Yi-Xia Jia
|
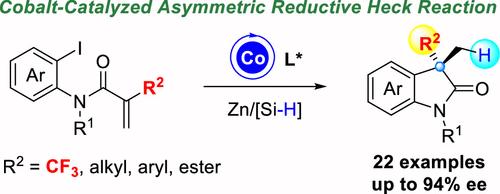
Herein, a cobalt-catalyzed intramolecular enantioselective reductive Heck reaction is disclosed. Starting from N-ortho-iodoaryl-2-(trifluoromethyl)acrylamides, a plethora of chiral oxindoles bearing trifluoromethylated quaternary stereogenic centers at the C3-position are achieved in moderate to good yields (up to 88% yield) and good to excellent enantioselectivities (up to 94% ee) by employing zinc/silane as reducing agent. Other than the trifluoromethyl group, a number of chiral oxindoles bearing alkyl, aryl, and ester groups at C3-position were also obtained albeit in relatively lower enantioselectivities (68–78% ee).
中文翻译:

钴催化的分子内对映选择性还原 Heck 反应,用于合成手性 3-三氟甲基化氧吲哚
在此,公开了一种钴催化的分子内对映选择性还原 Heck 反应。从 N-邻碘芳基-2-(三氟甲基)丙烯酰胺开始,通过使用锌/硅烷作为还原剂,在 C3 位具有三氟甲基化季立体中心的大量手性氧吲哚以中等至良好的产率(高达 88% 的产率)和良好至极好的对映选择性(高达 94% ee)获得。除了三氟甲基外,还获得了许多在 C3 位带有烷基、芳基和酯基的手性氧吲哚,尽管对映选择性相对较低 (68-78% ee)。
更新日期:2024-11-20
中文翻译:

钴催化的分子内对映选择性还原 Heck 反应,用于合成手性 3-三氟甲基化氧吲哚
在此,公开了一种钴催化的分子内对映选择性还原 Heck 反应。从 N-邻碘芳基-2-(三氟甲基)丙烯酰胺开始,通过使用锌/硅烷作为还原剂,在 C3 位具有三氟甲基化季立体中心的大量手性氧吲哚以中等至良好的产率(高达 88% 的产率)和良好至极好的对映选择性(高达 94% ee)获得。除了三氟甲基外,还获得了许多在 C3 位带有烷基、芳基和酯基的手性氧吲哚,尽管对映选择性相对较低 (68-78% ee)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号