当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trifluoromethylthiolation Carbonylation of Unactivated Alkenes via Distal Migration
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.orglett.4c04151 Ren-Guan Miao, Yuanrui Wang, Zhi-Peng Bao, Xiao-Feng Wu
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.orglett.4c04151 Ren-Guan Miao, Yuanrui Wang, Zhi-Peng Bao, Xiao-Feng Wu
|
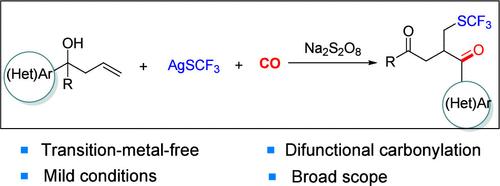
Sulfur-containing compounds represent a significant category of organic compounds, and the introduction of sulfur groups into organic compounds can effectively enhance their biological activity and synthetic diversity. Although a variety of difunctionalization reactions of alkenes based on sulfur radicals have been documented, significant challenges remain in the carbonylative difunctionalization of unactivated alkenes by the addition of a sulfur radical. Herein, we present a trifluoromethylthiolative carbonylation reaction of unactivated alkenes, which goes through the addition of a trifluoromethylthiol radical to unactivated alkenes and then carbonylation of the newly generated carbon radical intermediate. A heterocyclic/aryl migration in the presence of carbon monoxide is crucial for the success of this methodology and finally resulted in the formation of sulfur-containing carbonylated products in good yields.
中文翻译:

三氟甲基硫醇化未活化烯烃的羰基化反应,通过远端迁移
含硫化合物是一类重要的有机化合物,在有机化合物中引入硫基团可以有效增强其生物活性和合成多样性。尽管已经记录了基于硫自由基的烯烃的各种二官能化反应,但通过添加硫自由基对未活化的烯烃进行羰基化二官能化仍然存在重大挑战。在此,我们提出了未活化烯烃的三氟甲基硫醇化羰基化反应,该反应通过向未活化烯烃添加三氟甲基硫醇自由基,然后对新生成的碳自由基中间体进行羰基化。在一氧化碳存在下,杂环/芳基迁移对于该方法的成功至关重要,并最终以良好的收率形成含硫的羰基化产物。
更新日期:2024-11-20
中文翻译:

三氟甲基硫醇化未活化烯烃的羰基化反应,通过远端迁移
含硫化合物是一类重要的有机化合物,在有机化合物中引入硫基团可以有效增强其生物活性和合成多样性。尽管已经记录了基于硫自由基的烯烃的各种二官能化反应,但通过添加硫自由基对未活化的烯烃进行羰基化二官能化仍然存在重大挑战。在此,我们提出了未活化烯烃的三氟甲基硫醇化羰基化反应,该反应通过向未活化烯烃添加三氟甲基硫醇自由基,然后对新生成的碳自由基中间体进行羰基化。在一氧化碳存在下,杂环/芳基迁移对于该方法的成功至关重要,并最终以良好的收率形成含硫的羰基化产物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号