当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereo- and Enantioselective Syntheses of 1,2-Oxaborinan-3-enes and δ-Boryl-Substituted Homoallylic Alcohols
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.orglett.4c03755 Zheye Zhang, Ming Chen
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.orglett.4c03755 Zheye Zhang, Ming Chen
|
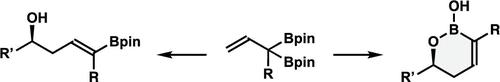
Stereo- and enantioselective syntheses of 1,2-oxaborinan-3-enes and δ-boryl-substituted homoallylic alcohols are reported. We developed a practical approach to synthesize α-boryl-substituted allylboronate. This reagent was utilized to generate α,α-disubstituted allylboronates, and such reagents cannot be accessed via the Pd-catalyzed alkene isomerization approach. Chiral Brønsted-acid-catalyzed aldehyde addition with these reagents gave 1,2-oxaborinan-3-enes with excellent stereo- and enantioselectivities. Lewis-acid-catalyzed aldehyde addition also worked well, affording δ-boryl-substituted homoallylic alcohols with high stereoselectivities. The enantioselective variant of the reaction was achieved via a chiral Brønsted-acid-catalyzed aldehyde addition and Pd-catalyzed alkene isomerization approach.
中文翻译:

1,2-氧杂基聚糖-3-烯和 δ-硼基取代的均烯丙基醇的立体和对映选择性合成
报道了 1,2-氧杂基聚糖-3-烯和 δ-硼基取代的均烯丙基醇的立体和对映选择性合成。我们开发了一种合成 α-硼基取代烯丙基硼酸盐的实用方法。该试剂用于生成 α,α-二取代烯丙基硼酸盐,并且此类试剂无法通过 Pd 催化的烯烃异构化方法获得。与这些试剂进行手性 Brønsted 酸催化的醛加成反应,得到具有优异立体选择性和对映选择性的 1,2-氧杂基聚糖-3-烯。路易斯酸催化的醛加成反应也很好,获得了具有高立体选择性的 δ-硼基取代的均烯丙醇。反应的对映选择性变体是通过手性 Brønsted 酸催化的醛加成和 Pd 催化的烯烃异构化方法实现的。
更新日期:2024-11-20
中文翻译:

1,2-氧杂基聚糖-3-烯和 δ-硼基取代的均烯丙基醇的立体和对映选择性合成
报道了 1,2-氧杂基聚糖-3-烯和 δ-硼基取代的均烯丙基醇的立体和对映选择性合成。我们开发了一种合成 α-硼基取代烯丙基硼酸盐的实用方法。该试剂用于生成 α,α-二取代烯丙基硼酸盐,并且此类试剂无法通过 Pd 催化的烯烃异构化方法获得。与这些试剂进行手性 Brønsted 酸催化的醛加成反应,得到具有优异立体选择性和对映选择性的 1,2-氧杂基聚糖-3-烯。路易斯酸催化的醛加成反应也很好,获得了具有高立体选择性的 δ-硼基取代的均烯丙醇。反应的对映选择性变体是通过手性 Brønsted 酸催化的醛加成和 Pd 催化的烯烃异构化方法实现的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号