当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
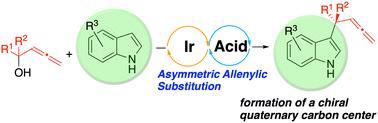
Iridium-catalyzed asymmetric allenylic substitution of tertiary racemic allenylic alcohols with indoles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-20 , DOI: 10.1039/d4qo01671h Takahiro Sawano, Manami Kobayashi, Momoko Ishikawa, Eri Ishikawa, Ryo Takeuchi
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-20 , DOI: 10.1039/d4qo01671h Takahiro Sawano, Manami Kobayashi, Momoko Ishikawa, Eri Ishikawa, Ryo Takeuchi
|
We report an allenylic substitution of racemic tertiary allenylic alcohols with indoles by a cooperative catalyst consisting of iridium/(P,olefin)-ligand catalyst and Lewis acid to give allenylated indoles bearing a quaternary carbon center with good enantioselectivities. 7-Azaindoles also reacted with allenylic alcohols to form the corresponding allenylated products with high enantioselectivities.
中文翻译:

铱催化的叔外消旋烯醇与吲哚的不对称烯基取代
我们报道了由铱/(P,烯烃)-配体催化剂和路易斯酸组成的协同催化剂用吲哚取代外消旋叔烯醛的烯基化吲哚,得到带有季碳中心的烯基化吲哚,具有良好的对映选择性。7-氮杂吲哚还与烯丙醇反应,形成相应的具有高对映选择性的烯基化产物。
更新日期:2024-11-20
中文翻译:

铱催化的叔外消旋烯醇与吲哚的不对称烯基取代
我们报道了由铱/(P,烯烃)-配体催化剂和路易斯酸组成的协同催化剂用吲哚取代外消旋叔烯醛的烯基化吲哚,得到带有季碳中心的烯基化吲哚,具有良好的对映选择性。7-氮杂吲哚还与烯丙醇反应,形成相应的具有高对映选择性的烯基化产物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号