Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncovering metabolic signatures in cancer-derived exosomes: LC-MS/MS and NMR profiling
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-20 , DOI: 10.1039/d4nr03454f Nandini Bajaj, Deepika Sharma
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-20 , DOI: 10.1039/d4nr03454f Nandini Bajaj, Deepika Sharma
|
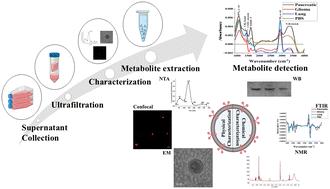
Understanding the intricate interplay between cancer metabolism and intercellular communication within the tumour microenvironment (TME) is crucial for advancing cancer diagnostics and therapeutics. In this study, we investigate the metabolites present in exosomes derived from three distinct cancer cell lines: pancreatic cancer (MiaPaCa-2), lung cancer (A549), and glioma (C6). Exosomes were isolated using ultrafiltration and characterized using a combination of techniques including nanoparticle tracking analysis (NTA), electron microscopy (EM), western blotting (WB) and Fourier-transform infrared (FTIR) spectroscopy. Leveraging state-of-the-art metabolomics techniques, including untargeted LC-MS/MS and NMR analyses, we elucidated the metabolic signatures encapsulated within cancer-derived exosomes. Notably, our investigation represents the first exploration of exosomal metabolites from pancreatic and glioma cells, addressing a significant gap in current knowledge. Furthermore, our study investigates the correlation between metabolites derived from different cancer cells, shedding light on potential metabolic interactions within the TME. Through comprehensive analyses, this study provides insights into dysregulated metabolic pathways driving cancer progression and offers novel perspectives on the diagnostic and therapeutic utility of exosomal metabolites. Importantly, common metabolites identified among cancer types suggest potential markers detectable by multiple techniques, enhancing their clinical applicability.
中文翻译:

揭示癌症来源的外泌体中的代谢特征:LC-MS/MS 和 NMR 分析
了解肿瘤微环境 (TME) 中癌症代谢和细胞间通讯之间错综复杂的相互作用对于推进癌症诊断和治疗至关重要。在这项研究中,我们研究了来自三种不同癌细胞系的外泌体中存在的代谢物:胰腺癌 (MiaPaCa-2)、肺癌 (A549) 和神经胶质瘤 (C6)。使用超滤分离外泌体,并使用纳米颗粒跟踪分析 (NTA)、电子显微镜 (EM)、蛋白质印迹 (WB) 和傅里叶变换红外 (FTIR) 光谱等技术的组合进行表征。利用最先进的代谢组学技术,包括非靶向 LC-MS/MS 和 NMR 分析,我们阐明了癌症衍生外泌体中封装的代谢特征。值得注意的是,我们的研究代表了对胰腺和神经胶质瘤细胞外泌体代谢物的首次探索,解决了当前知识的重大差距。此外,我们的研究调查了来自不同癌细胞的代谢物之间的相关性,揭示了 TME 内潜在的代谢相互作用。通过全面分析,本研究提供了对驱动癌症进展的失调代谢途径的见解,并为外泌体代谢物的诊断和治疗效用提供了新的视角。重要的是,在癌症类型中鉴定的常见代谢物表明可通过多种技术检测到的潜在标志物,从而提高了它们的临床适用性。
更新日期:2024-11-20
中文翻译:

揭示癌症来源的外泌体中的代谢特征:LC-MS/MS 和 NMR 分析
了解肿瘤微环境 (TME) 中癌症代谢和细胞间通讯之间错综复杂的相互作用对于推进癌症诊断和治疗至关重要。在这项研究中,我们研究了来自三种不同癌细胞系的外泌体中存在的代谢物:胰腺癌 (MiaPaCa-2)、肺癌 (A549) 和神经胶质瘤 (C6)。使用超滤分离外泌体,并使用纳米颗粒跟踪分析 (NTA)、电子显微镜 (EM)、蛋白质印迹 (WB) 和傅里叶变换红外 (FTIR) 光谱等技术的组合进行表征。利用最先进的代谢组学技术,包括非靶向 LC-MS/MS 和 NMR 分析,我们阐明了癌症衍生外泌体中封装的代谢特征。值得注意的是,我们的研究代表了对胰腺和神经胶质瘤细胞外泌体代谢物的首次探索,解决了当前知识的重大差距。此外,我们的研究调查了来自不同癌细胞的代谢物之间的相关性,揭示了 TME 内潜在的代谢相互作用。通过全面分析,本研究提供了对驱动癌症进展的失调代谢途径的见解,并为外泌体代谢物的诊断和治疗效用提供了新的视角。重要的是,在癌症类型中鉴定的常见代谢物表明可通过多种技术检测到的潜在标志物,从而提高了它们的临床适用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号