当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Does the active surface area determine the activity of silica supported nickel catalysts in CO2 methanation reaction?
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157827 Karolina Karpińska-Wlizło, Witold Zawadzki, Grzegorz Słowik, Wojciech Gac
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157827 Karolina Karpińska-Wlizło, Witold Zawadzki, Grzegorz Słowik, Wojciech Gac
|
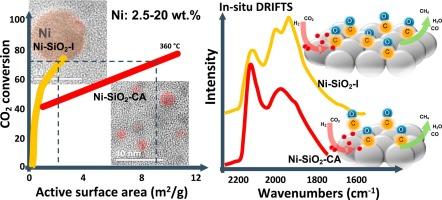
Two series of silica supported catalysts with comparable nickel contents varying from 2.5 to 20 wt.% were prepared by wet impregnation method in the absence and presence of citric acid in the impregnation solution. Temperature-programmed reduction, X-ray diffraction and electron microscopy studies indicated changes of reducibility of nickel oxide species in the corresponding catalysts and formation of nickel nanoparticles of different size and morphology. A gradual increase in the performance of catalysts in the CO2 methanation reaction was observed with increasing Ni loading. The application of a modified impregnation method led to a reduction in the size of the nickel crystallites, which increased the active surface area of the catalysts, improving their activity and selectivity towards methane at low temperatures, as well as their stability at high temperatures. It was shown that the high active surface area of silica-supported nickel catalysts, due to the presence of small crystallites, is a key factor in increasing their activity. However, other catalyst properties may also play an important role. Hydrogen temperature-programmed desorption and in-situ DRIFTS adsorption/desorption of CO, CO2 and CO2 hydrogenation reaction studies indicated that modification of the method of catalyst synthesis led to changes in the surface properties of the catalysts, affecting the way CO2 and H2 activation and the transformation of resulted intermediate species to the final reaction products.
中文翻译:

活性表面积是否决定了二氧化硅负载的镍催化剂在 CO2 甲烷化反应中的活性?
在浸渍溶液中不存在柠檬酸的情况下,通过湿浸渍法制备了两个系列的二氧化硅负载催化剂,镍含量从 2.5 到 20 wt.% 不等。程序升温还原、X 射线衍射和电子显微镜研究表明,相应催化剂中氧化镍物质的还原性发生了变化,并形成了不同尺寸和形态的纳米镍颗粒。随着 Ni 负载量的增加,观察到催化剂在 CO2 甲烷化反应中的性能逐渐提高。改进的浸渍方法的应用导致镍微晶的尺寸减小,这增加了催化剂的活性表面积,提高了它们在低温下对甲烷的活性和选择性,以及它们在高温下的稳定性。结果表明,由于存在小微晶,二氧化硅负载镍催化剂的高活性表面积是提高其活性的关键因素。然而,其他催化剂特性也可能起重要作用。氢气程序升温解吸和 CO、CO2 和 CO2 加氢反应的原位 DRIFTS 吸附/解吸研究表明,催化剂合成方法的修改导致催化剂表面性质的变化,影响 CO2 和 H2 活化的方式以及所得中间体向最终反应产物的转化。
更新日期:2024-11-19
中文翻译:

活性表面积是否决定了二氧化硅负载的镍催化剂在 CO2 甲烷化反应中的活性?
在浸渍溶液中不存在柠檬酸的情况下,通过湿浸渍法制备了两个系列的二氧化硅负载催化剂,镍含量从 2.5 到 20 wt.% 不等。程序升温还原、X 射线衍射和电子显微镜研究表明,相应催化剂中氧化镍物质的还原性发生了变化,并形成了不同尺寸和形态的纳米镍颗粒。随着 Ni 负载量的增加,观察到催化剂在 CO2 甲烷化反应中的性能逐渐提高。改进的浸渍方法的应用导致镍微晶的尺寸减小,这增加了催化剂的活性表面积,提高了它们在低温下对甲烷的活性和选择性,以及它们在高温下的稳定性。结果表明,由于存在小微晶,二氧化硅负载镍催化剂的高活性表面积是提高其活性的关键因素。然而,其他催化剂特性也可能起重要作用。氢气程序升温解吸和 CO、CO2 和 CO2 加氢反应的原位 DRIFTS 吸附/解吸研究表明,催化剂合成方法的修改导致催化剂表面性质的变化,影响 CO2 和 H2 活化的方式以及所得中间体向最终反应产物的转化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号