当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vanadium Substitution Dictates H Atom Uptake at Lindqvist-type Polyoxotungstates
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.inorgchem.4c03873 Dominic Shiels, Zhou Lu, Magda Pascual-Borràs, Nathalia Cajiao, Thompson V. Marinho, William W. Brennessel, Michael L. Neidig, R. John Errington, Ellen M. Matson
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.inorgchem.4c03873 Dominic Shiels, Zhou Lu, Magda Pascual-Borràs, Nathalia Cajiao, Thompson V. Marinho, William W. Brennessel, Michael L. Neidig, R. John Errington, Ellen M. Matson
|
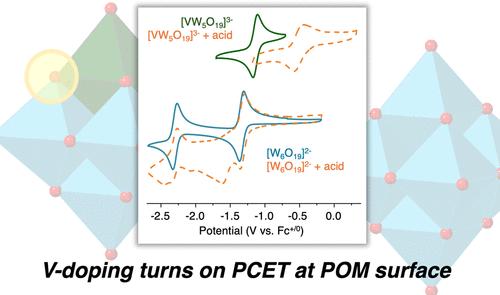
Understanding how modification of molecular structures changes the thermochemistry of H atom uptake can provide design criteria for the formation of highly active catalysts for reductive transformations. Herein, we describe the effect of doping an atomically precise polyoxotungstate with vanadium on proton-coupled electron transfer (PCET) reactivity. The Lindqvist-type polyoxotungstate [W6O19]2– displays reversible redox chemistry, which was found to be unchanged in the presence of acid, indicating an inability to couple reduction with protonation. However, the incorporation of a single vanadium center into the structure significantly changes the reactivity, and the potential required for one-electron reduction of [VW5O19]3– was shown to vary with the strength of the acid added. Construction of a potential-pKa diagram allowed assessment of the thermodynamics of H atom uptake, indicating BDFE(O–H) ≈ 64 kcal/mol, while chemical synthesis of the reduced/protonated derivative (TBA)3[VW5O19H] was used to probe the position of protonation.
中文翻译:

钒取代决定了 Lindqvist 型多氧钨酸盐的 H 原子摄取
了解分子结构的修饰如何改变 H 原子摄取的热化学可以为形成用于还原转化的高活性催化剂提供设计标准。在本文中,我们描述了用钒掺杂原子精确的多氧钨酸盐对质子耦合电子转移 (PCET) 反应性的影响。Lindqvist 型多氧钨酸盐 [W6O19]2– 显示可逆氧化还原化学,发现在酸存在下保持不变,表明无法将还原与质子化耦合。然而,将单个钒中心掺入结构中会显着改变反应性,并且 [VW5O19]3– 的单电子还原所需的电位随添加的酸的强度而变化。构建电位 pKa 图可以评估 H 原子摄取的热力学,表明 BDFE(O-H) ≈ 64 kcal/mol,而还原/质子化衍生物 (TBA)3[VW5O19H] 的化学合成用于探测质子化的位置。
更新日期:2024-11-20
中文翻译:

钒取代决定了 Lindqvist 型多氧钨酸盐的 H 原子摄取
了解分子结构的修饰如何改变 H 原子摄取的热化学可以为形成用于还原转化的高活性催化剂提供设计标准。在本文中,我们描述了用钒掺杂原子精确的多氧钨酸盐对质子耦合电子转移 (PCET) 反应性的影响。Lindqvist 型多氧钨酸盐 [W6O19]2– 显示可逆氧化还原化学,发现在酸存在下保持不变,表明无法将还原与质子化耦合。然而,将单个钒中心掺入结构中会显着改变反应性,并且 [VW5O19]3– 的单电子还原所需的电位随添加的酸的强度而变化。构建电位 pKa 图可以评估 H 原子摄取的热力学,表明 BDFE(O-H) ≈ 64 kcal/mol,而还原/质子化衍生物 (TBA)3[VW5O19H] 的化学合成用于探测质子化的位置。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号