当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolobal Cationic Iridium Dihydride and Dizinc Complexes: A Dual Role for the ZnR Ligand Enhances H2 Activation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.inorgchem.4c04058 Amber M. Walsh, Lia Sotorrios, Rebecca G. Cameron, Anne-Frédérique Pécharman, Barbara Procacci, John P. Lowe, Stuart A. Macgregor, Mary F. Mahon, Neil T. Hunt, Michael K. Whittlesey
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-20 , DOI: 10.1021/acs.inorgchem.4c04058 Amber M. Walsh, Lia Sotorrios, Rebecca G. Cameron, Anne-Frédérique Pécharman, Barbara Procacci, John P. Lowe, Stuart A. Macgregor, Mary F. Mahon, Neil T. Hunt, Michael K. Whittlesey
|
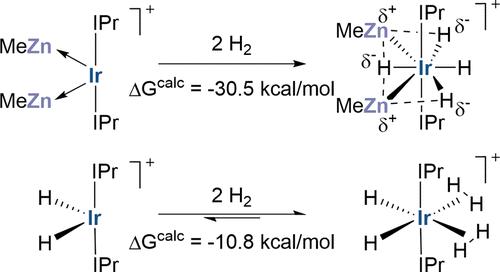
The reaction of [Ir(IPr)2H2][BArF4] (1; IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; BArF4 = B{C6H3(3,5-CF3)2}4) with ZnMe2 proceeds with CH4 elimination to give [Ir(IPr)(IPr′)(ZnMe)2H][BArF4] (3, where (IPr′) is a cyclometalated IPr ligand). 3 reacts with H2 to form tetrahydride [Ir(IPr)2(ZnMe)2H4][BArF4], 4, that loses H2 under forcing conditions to form [Ir(IPr)2(ZnMe)2H2][BArF4], 5. Crystallization of 3 also results in the formation of its noncyclometalated isomer, [Ir(IPr)2(ZnMe)2][BArF4], 2, in the solid state. Reactions of 1 and CdMe2 form [Ir(IPr)2(CdMe)2][BArF4], 6, and [Ir(IPr)(IPr′)(CdMe)2H][BArF4], 7, which reacts with H2 to give [Ir(IPr)2(CdMe)2H4][BArF4], 8, and [Ir(IPr)2(CdMe)2H2][BArF4], 9. Structures of 2–8 are determined crystallographically. Computational analyses show the various hydrides in 3–5 sit on a terminal to bridging continuum, with bridging hydrides exhibiting greater Znδ+···Hδ− electrostatic interaction. The isolobal analogy between H and ZnMe ligands holds when both are present as terminal ligands. However, the electrostatic component to the Znδ+···Hδ− unit renders it significantly different to a nominally isolobal H···H moiety. Thus, H2 addition to 3 is irreversible, whereas H2 addition to 1 reversibly forms highly fluxional [Ir(IPr)2(η2-H2)2H2][BArF4], 11. Computed mechanisms for cyclometalation and H2 addition showcase the role of the bridging Znδ+···Hδ− moiety in promoting reactivity. In this, the Lewis acidic ZnMe ligand plays a dual role: as a terminal Z-type ligand that can stabilize electron-rich Ir centers through direct Ir-ZnMe bonding, or by stabilizing strongly hydridic character via Znδ+···Hδ− interactions.
中文翻译:

等叶阳离子铱二氢化物和二锌配合物:ZnR 配体的双重作用增强 H2 活化
[Ir(IPr)2H2][BArF4] (1;IPr = 1,3-双(2,6-二异丙基苯基)咪唑-2-亚基;BArF4 = B{C6H3(3,5-CF3)2}4) 与 ZnMe2 一起消除 CH4,得到 [Ir(IPr)(IPr′)(ZnMe)2H][BArF4](3,其中 (IPr′) 是环金属化的 IPr 配体)。3 与 H2 反应生成四氢化物 [Ir(IPr)2(ZnMe)2H4][BArF4], 4,在强迫条件下失去 H2 生成 [Ir(IPr)2(ZnMe)2H2][BArF4], 5.3 的结晶也导致形成其非环金属异构体 [Ir(IPr)2(ZnMe)2][BArF4], 2,处于固态。1 和 CdMe2 的反应形成 [Ir(IPr)2(CdMe)2][BArF4]、6 和 [Ir(IPr)(IPr′)(CdMe)2H][BArF4], 7,它与 H2 反应生成 [Ir(IPr)2(CdMe)2H4][BArF4], 8 和 [Ir(IPr)2(CdMe)2H2][BArF4], 9. 2-8 的结构通过晶体学确定。计算分析表明,3-5 中的各种氢化物位于桥接连续体的末端,桥接氢化物表现出更大的 Znδ+···Hδ− 静电相互作用。 H 和 ZnMe 配体之间的等叶类比在两者都作为末端配体存在时成立。然而,静电组分对 Znδ+···Hδ− 单位使其与名义上的等叶 H···H 部分。因此,H2 加成 3 是不可逆的,而 H2 加成 1 可逆地形成高通量 [Ir(IPr)2(η2-H 2)2] [BArF4],11。计算的环金属化和 H2 加成机制展示了桥接 Znδ+··· 的作用Hδ− 促进反应性的部分。在这方面,Lewis 酸性 ZnMe 配体起着双重作用:作为末端 Z 型配体,可以通过直接 Ir-ZnMe 键合稳定富电子的 Ir 中心,或通过 Znδ+···Hδ− 交互作用。
更新日期:2024-11-20
中文翻译:

等叶阳离子铱二氢化物和二锌配合物:ZnR 配体的双重作用增强 H2 活化
[Ir(IPr)2H2][BArF4] (1;IPr = 1,3-双(2,6-二异丙基苯基)咪唑-2-亚基;BArF4 = B{C6H3(3,5-CF3)2}4) 与 ZnMe2 一起消除 CH4,得到 [Ir(IPr)(IPr′)(ZnMe)2H][BArF4](3,其中 (IPr′) 是环金属化的 IPr 配体)。3 与 H2 反应生成四氢化物 [Ir(IPr)2(ZnMe)2H4][BArF4], 4,在强迫条件下失去 H2 生成 [Ir(IPr)2(ZnMe)2H2][BArF4], 5.3 的结晶也导致形成其非环金属异构体 [Ir(IPr)2(ZnMe)2][BArF4], 2,处于固态。1 和 CdMe2 的反应形成 [Ir(IPr)2(CdMe)2][BArF4]、6 和 [Ir(IPr)(IPr′)(CdMe)2H][BArF4], 7,它与 H2 反应生成 [Ir(IPr)2(CdMe)2H4][BArF4], 8 和 [Ir(IPr)2(CdMe)2H2][BArF4], 9. 2-8 的结构通过晶体学确定。计算分析表明,3-5 中的各种氢化物位于桥接连续体的末端,桥接氢化物表现出更大的 Znδ+···Hδ− 静电相互作用。 H 和 ZnMe 配体之间的等叶类比在两者都作为末端配体存在时成立。然而,静电组分对 Znδ+···Hδ− 单位使其与名义上的等叶 H···H 部分。因此,H2 加成 3 是不可逆的,而 H2 加成 1 可逆地形成高通量 [Ir(IPr)2(η2-H 2)2] [BArF4],11。计算的环金属化和 H2 加成机制展示了桥接 Znδ+··· 的作用Hδ− 促进反应性的部分。在这方面,Lewis 酸性 ZnMe 配体起着双重作用:作为末端 Z 型配体,可以通过直接 Ir-ZnMe 键合稳定富电子的 Ir 中心,或通过 Znδ+···Hδ− 交互作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号