当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Spectroscopic Investigations of pH-Dependent Mo(V) Species in a Bacterial Sulfite-Oxidizing Enzyme
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.inorgchem.4c02584 Ahmed Djeghader, Julia Rendon, Frédéric Biaso, Guillaume Gerbaud, Wolfgang Nitschke, Barbara Schoepp-Cothenet, Tewfik Soulimane, Stéphane Grimaldi
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.inorgchem.4c02584 Ahmed Djeghader, Julia Rendon, Frédéric Biaso, Guillaume Gerbaud, Wolfgang Nitschke, Barbara Schoepp-Cothenet, Tewfik Soulimane, Stéphane Grimaldi
|
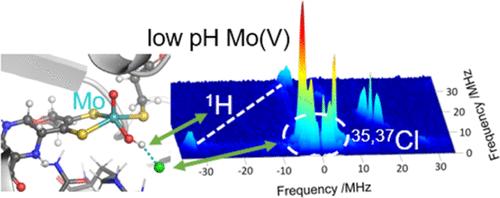
Mono-pyranopterin-containing sulfite-oxidizing enzymes (SOEs), including eukaryotic sulfite oxidases and homologous prokaryotic sulfite dehydrogenases (SDHs), are molybdenum enzymes that exist in almost all forms of life, where they catalyze the direct oxidation of sulfite into sulfate, playing a key role in protecting cells and organisms against sulfite-induced damage. To decipher their catalytic mechanism, we have previously provided structural and spectroscopic evidence for direct coordination of HPO42– to the Mo atom at the active site of the SDH from the hyperthermophilic bacterium Thermus thermophilus (TtSDH), mimicking the proposed sulfate-bound intermediate proposed to be formed during catalysis. In this work, by solving the X-ray crystallographic structure of the unbound enzyme, we resolve the changes in the hydrogen bonding network in the molybdenum environment that enable the stabilization of the previously characterized phosphate adduct. In addition, electron paramagnetic resonance spectroscopic study of the enzyme over a wide pH range reveals the formation of pH-dependent Mo(V) species, a characteristic feature of eukaryotic SOEs. The combined use of HYSCORE, H2O/D2O exchange, and density functional theory calculations allows the detailed characterization of a typical low pH Mo(V) species previously unreported in bacterial SOEs, underlining the conservation of the active site properties of SOEs irrespective of their source organism.
中文翻译:

细菌亚硫酸盐氧化酶中 pH 依赖性 Mo(V) 物质的结构和光谱研究
含单吡喃呤的亚硫酸盐氧化酶 (SOE),包括真核亚硫酸盐氧化酶和同源原核亚硫酸盐脱氢酶 (SDH),是存在于几乎所有生命形式的钼酶,它们催化亚硫酸盐直接氧化成硫酸盐,在保护细胞和生物体免受亚硫酸盐诱导的损伤中起关键作用。为了破译它们的催化机制,我们之前提供了结构和光谱证据,证明 HPO42– 与超嗜热细菌嗜热热菌 (TtSDH) 在 SDH 活性位点的 Mo 原子直接配位,模拟了拟议的硫酸盐结合中间体在催化过程中形成。在这项工作中,通过解析未结合酶的 X 射线晶体结构,我们解决了钼环境中氢键网络的变化,从而能够稳定先前表征的磷酸盐加合物。此外,在较宽的 pH 范围内对酶进行电子顺磁共振光谱研究揭示了 pH 依赖性 Mo(V) 物质的形成,这是真核 SOE 的一个特征。HYSCORE、H2O/D2O 交换和密度泛函理论计算的结合允许对以前在细菌 SOE 中未报道的典型低 pH Mo(V) 物种进行详细表征,强调 SOE 的活性位点特性的保留,无论其来源生物体如何。
更新日期:2024-11-20
中文翻译:

细菌亚硫酸盐氧化酶中 pH 依赖性 Mo(V) 物质的结构和光谱研究
含单吡喃呤的亚硫酸盐氧化酶 (SOE),包括真核亚硫酸盐氧化酶和同源原核亚硫酸盐脱氢酶 (SDH),是存在于几乎所有生命形式的钼酶,它们催化亚硫酸盐直接氧化成硫酸盐,在保护细胞和生物体免受亚硫酸盐诱导的损伤中起关键作用。为了破译它们的催化机制,我们之前提供了结构和光谱证据,证明 HPO42– 与超嗜热细菌嗜热热菌 (TtSDH) 在 SDH 活性位点的 Mo 原子直接配位,模拟了拟议的硫酸盐结合中间体在催化过程中形成。在这项工作中,通过解析未结合酶的 X 射线晶体结构,我们解决了钼环境中氢键网络的变化,从而能够稳定先前表征的磷酸盐加合物。此外,在较宽的 pH 范围内对酶进行电子顺磁共振光谱研究揭示了 pH 依赖性 Mo(V) 物质的形成,这是真核 SOE 的一个特征。HYSCORE、H2O/D2O 交换和密度泛函理论计算的结合允许对以前在细菌 SOE 中未报道的典型低 pH Mo(V) 物种进行详细表征,强调 SOE 的活性位点特性的保留,无论其来源生物体如何。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号