当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct, Acid-Free Uranium Purification from Solid Analytical Waste by Supercritical Carbon Dioxide Extraction with Incinerable N,N-Dialkyl Amide Adducts
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.iecr.4c03075 Avinash S. Kanekar, Shiny S. Kumar, Ankita Rao, Sangita Dhara
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-19 , DOI: 10.1021/acs.iecr.4c03075 Avinash S. Kanekar, Shiny S. Kumar, Ankita Rao, Sangita Dhara
|
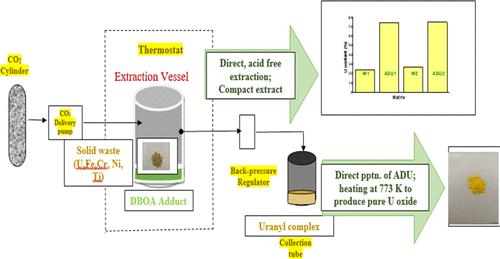
Sustainable method development for uranium recovery from secondary lean sources (scraps, waste matrices) is a major thrust area for achieving net zero by the nuclear industry. Herein we demonstrated a simple, two-step crude solid (analytical waste, U content ∼24%) to pure solid (ammonium diuranate, near-stoichiometric U content ∼74%) transformation aided by direct, acid-free supercritical carbon dioxide (SC CO2) extraction followed by uranium precipitation from the compact extract. Augmenting the green quotient, this is the first systematic study with incinerable N,N-dialkyl amide adducts, providing insight into relevant physical properties (density and viscosity variation with temperature) as well as exploring the relation between their composition (nitric acid and water content) and uranium extraction-purification. Temperature and pressure affected the SC CO2 extraction efficiency by influencing the solubility of metal-amide complexes. In comparison to adducts of other amides and tributyl phosphate (TBP), N,N-dibutyl octanamide (DBOA) yielded the highest uranium extraction (>88%) and purification with the highest separation factors with respect to metal ion impurities, which were further enhanced with a higher matrix amount. Spectral examination (IR, UV–vis) revealed the nature of the uranium-amide complex in the SC CO2 extract. Ammonium diuranate, obtained from direct precipitation from the concentrated SC CO2 extract, could be heated to 773 K for uranium oxide production. A comparative study of the present scheme with the multistep, chemical-intensive conventional route (overall ∼86% U extraction efficiency, SFFe ∼ 19) highlighted highly efficient uranium extraction-purification (overall ∼88% U extraction efficiency, SFFe ∼ 46) by employing the green amide adduct with minimization of process steps (single-step dissolution-complexation).
中文翻译:

使用可焚烧的 N,N-二烷基酰胺加合物进行超临界二氧化碳萃取,从固体分析废料中直接纯化无酸铀
从次级贫源(废料、废料基质)中回收铀的可持续方法开发是核工业实现净零排放的主要推动领域。在此,我们展示了一种简单的两步粗固体(分析废液,铀含量 ∼24%)到纯固体(二铀酸铵,近化学计量铀含量 ∼74%)的转化,通过直接、无酸的超临界二氧化碳 (SC CO2) 萃取,然后从致密提取物中沉淀铀。这是第一项使用可焚烧 N,N-二烷基酰胺加合物的系统研究,提供了对相关物理性质(密度和粘度随温度变化)的见解,并探索了它们的组成(硝酸和水含量)与铀提取-纯化之间的关系。温度和压力通过影响金属-酰胺复合物的溶解度来影响 SC CO2 提取效率。与其他酰胺和磷酸三丁酯 (TBP) 的加合物相比,N,N-二丁基辛酰胺 (DBOA) 在金属离子杂质方面产生最高的铀提取 (>88%) 和纯化,具有最高的分离因子,随着基质含量的增加,这些分离因子进一步增强。光谱检查(IR、UV-Vis)揭示了 SC CO2 提取物中铀酰胺络合物的性质。从浓缩的 SC CO2 提取物中直接沉淀获得的二铀酸铵可以加热到 773 K 以生产氧化铀。 将本方案与多步骤、化学密集型常规路线(总体 ∼86% 铀萃取效率,SFFe ∼ 19)进行比较研究突出了高效的铀萃取-提纯(总体∼88% 铀萃取效率,SFFe ∼ 46)通过采用绿色酰胺加合物,最大限度地减少了工艺步骤(单步溶解-络合)。
更新日期:2024-11-19
中文翻译:

使用可焚烧的 N,N-二烷基酰胺加合物进行超临界二氧化碳萃取,从固体分析废料中直接纯化无酸铀
从次级贫源(废料、废料基质)中回收铀的可持续方法开发是核工业实现净零排放的主要推动领域。在此,我们展示了一种简单的两步粗固体(分析废液,铀含量 ∼24%)到纯固体(二铀酸铵,近化学计量铀含量 ∼74%)的转化,通过直接、无酸的超临界二氧化碳 (SC CO2) 萃取,然后从致密提取物中沉淀铀。这是第一项使用可焚烧 N,N-二烷基酰胺加合物的系统研究,提供了对相关物理性质(密度和粘度随温度变化)的见解,并探索了它们的组成(硝酸和水含量)与铀提取-纯化之间的关系。温度和压力通过影响金属-酰胺复合物的溶解度来影响 SC CO2 提取效率。与其他酰胺和磷酸三丁酯 (TBP) 的加合物相比,N,N-二丁基辛酰胺 (DBOA) 在金属离子杂质方面产生最高的铀提取 (>88%) 和纯化,具有最高的分离因子,随着基质含量的增加,这些分离因子进一步增强。光谱检查(IR、UV-Vis)揭示了 SC CO2 提取物中铀酰胺络合物的性质。从浓缩的 SC CO2 提取物中直接沉淀获得的二铀酸铵可以加热到 773 K 以生产氧化铀。 将本方案与多步骤、化学密集型常规路线(总体 ∼86% 铀萃取效率,SFFe ∼ 19)进行比较研究突出了高效的铀萃取-提纯(总体∼88% 铀萃取效率,SFFe ∼ 46)通过采用绿色酰胺加合物,最大限度地减少了工艺步骤(单步溶解-络合)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号